MAIA Biotechnology: Significantly Undervalued with Novel Mechanism and Impressive Early Data
Asymmetric high-risk/high-reward; worthy of at least a speculative position.
Key Points
Lead asset THIO has a unique telomere-targeting mechanism and has reported impressive preliminary results in NSCLC.
THIO's 90%+ disease control rate in second- and third-line NSCLC is an improvement over current standard of care.
Plans to pursue accelerated approval in NSCLC in 2025 and has the potential to become a new standard of care.
Has a clinical supply agreement with Regeneron. Continued positive results may result in an extension with Regeneron or other strategic partnerships.
Intro
MAIA Biotechnology (MAIA), a small 2022 biotech IPO, has reported encouraging preliminary Phase 2 data in non-small cell lung cancer (NSCLC) that suggests its lead asset, THIO, could represent a fundamental advancement in the treatment of solid tumors. THIO’s unique telomere-targeting mechanism of action selectively kills telomerase-expressing cancer cells, and was found in preclinical studies to be synergistic with immune checkpoint inhibitor (ICI) therapies (e.g. Keytruda). In our view, the market’s lack of awareness of MAIA has resulted in a dramatically asymmetric investment opportunity as MAIA sits on the cusp of potentially demonstrating a deep and durable efficacy profile in 2024.
As of the most recent data cutoff in September 2023, THIO’s ~90%+ disease control rates (DCRs), which includes patients with tumor shrinkage or stable disease (SD), are outpacing standard of care (SoC) in both second- and third-line NSCLC. Management also recently indicated that overall response rates (ORRs; 30%+ tumor shrinkage) for THIO’s recently-selected registrational dose of 180 mg are tracking to 35%+, which would represent a significant improvement over standard of care in second- and third-line patients. The company is expected to provide a data update at the JPM Healthcare Conference next week and plans to pursue accelerated approval in NSCLC in 2025 pending continued efficacy.
In its ongoing Phase 2 trial, THIO is dosed in combination with Regeneron’s PD-1 ICI Libtayo (cemiplimab), for which it has a clinical supply agreement in NSCLC. An extension (or expansion) of this agreement is another potential upcoming catalyst, as is a partnership with another big pharma oncology player in other cancer indications, perhaps in one of THIO’s three orphan drug designated (ODD) indications, as THIO’s data continues to mature.
With an elegant mechanism, remarkably benign safety profile for oncology, and efficacy data showing preliminary signs of superiority versus current standard of care, THIO’s near-term upside scenario is to become a breakthrough in first-line refractory NSCLC patients, with longer-term potential to move to other solid tumor types and earlier lines of therapy. At a tiny ~$20 million of market cap, we believe MAIA currently presents a large asymmetric opportunity that is worth at least a speculative position as an oncology therapy that could begin to illustrate a fundamentally differentiated clinical profile in the coming weeks and months.
THIO and Science
THIO (6-thio-dG) was originally developed and researched at the NIH in the 1970s as a chemotherapy in multiple tumor types (at much higher doses than MAIA is currently testing), but was abandoned due to mixed results. THIO sat dormant for decades while the scientific understanding of telomeres came into focus in the 1990’s and early 2000’s. In the mid-2010’s, THIO resumed study by Dr. Jerry Shay’s lab at UT Southwestern, which demonstrated that THIO induced telomere dysfunction (Mender et al., 2015). Dr. Ilgen Mender, a researcher at Dr. Shay’s lab, published the seminal paper describing THIO’s mechanism of action in Cancer Cell in 2020 (Mender, et al., 2020).
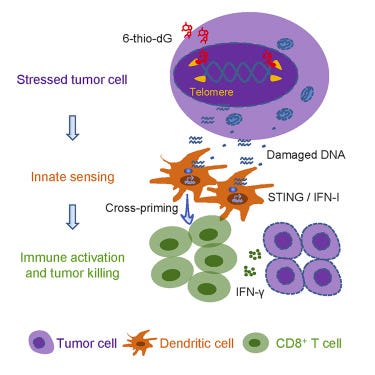
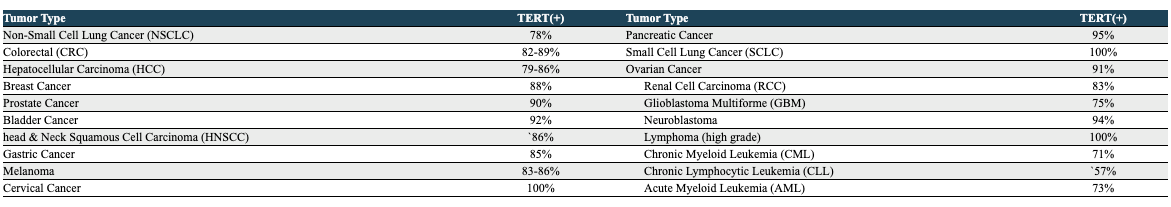
THIO is a nucleoside analog that is incorporated into telomeres by the enzyme telomerase. Telomeres are the “caps” on the ends of our DNA that protect it from damage and mutations during cell division and mitophagy; telomeres are built and maintained by the enzyme telomerase. In humans, telomerase is primarily expressed in the early stages of development, and is only expressed in a select few cell types in adulthood (mostly plasma cells). In almost all cancers, however, telomerase expression is reactivated, allowing cancer cells to reach a state of “replicative immortality”, allowing them to divide uncontrollably and indefinitely. Telomerase has been found to be expressed in 80%+ of NSCLC tumors.
As a nucleoside (building block of DNA) analog, THIO is incorporated directly into the telomere by telomerase, where it causes instability and unraveling of the DNA, causing cell death. Dr. Mender’s 2020 paper also characterized THIO’s immunogenic effect, in which the damage caused to telomerase-expressing cells is sensed by the innate immune system, eventually mounting an adaptive (T cell) immune response. This allows THIO to sensitize the immune system to immunologically “cold” (unrecognized) tumors, as well as overcome PD-L1 inhibitor resistance.
THIO’s telomere-targeting mechanism is not a new approach in oncology. Geron’s lead candidate, imetelstat, is a telomerase enzyme inhibitor. THIO’s mechanism is superior to imetelstat because inhibition of telomerase still allows the cell to divide until it has run down the existing telomere, whereas THIO actively kills telomerase-expressing cells. MAIA’s Chief Scientific Officer, Dr. Sergei Gryaznov, was the co-inventor of imetelstat and is a world-renowned leader in telomere research.
Preclinical Data
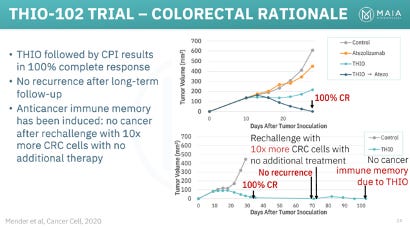
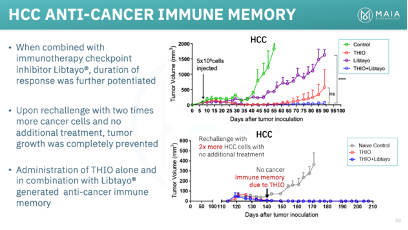
Between MAIA and Dr. Shay’s lab, THIO has demonstrated impressive preclinical results in a wide range of solid tumor types and in combination with multiple ICIs (Libtayo, Keytruda, Tecentriq). We plan to explore THIOs potential in various non-NSCLC in a future article, but will highlight its particularly impressive effects in colorectal cancer and hepatocellular carcinoma (HCC). In mouse models of both diseases, THIO was able to deliver 100% complete response rates (complete tumor eradication).
THIOs preclinical data led the FDA to grant it Orphan Drug Designation in both HCC and small cell lung cancer (SCLC) in 2022, and more recently, glioblastoma.
Additionally, THIO’s selective activity in telomerase-expressing cells has also led to a remarkably innocuous safety profile, especially in the oncology space. Translated to humans, THIO’s curative dose in mice was 60 mg per cycle, while the maximum tolerated dose in humans has been established at 2,500 mg per cycle, lending a very large therapeutic index. In the ongoing Phase 2 trial, THIO-101, the most common Grade 3+ side effects, which were elevated liver enzymes, were reported in just 9% of patients.
(Additional info: Aubrey de Grey, a marked scientific author, researcher, and thought leader in biology and longevity spaces, is apparently aware of MAIA and THIO and regards THIO as “the single most interesting thing in oncology right now”. He can be found discussing THIO on two little-watched podcasts here (minute 31:00) and here.
Regeneron Agreement
Based on THIO’s preclinical data, MAIA was able to secure a clinical supply agreement with Regeneron, under which Regeneron provides MAIA with Libtayo for the duration of the THIO-101 trial. The deal lends Regeneron exclusivity to THIO only in NSCLC and only among ICIs. Based on the planned patients for THIO-101, the company estimates that the Regeneron deal is worth $32 million of clinical supply of Libtayo.
As the agreement does not extend past THIO-101, we would assume that MAIA’s negotiations with Regeneron for an extension to a Phase 3/confirmatoty trial are currently ongoing. If Regeneron is going to extend the partnership (which would appear likely given the results thus far), we would expect an announcement in the coming months, perhaps following the JPM data update next week, or after ASCO in June at the latest.
Regeneron sales of Libtayo have underperformed expectations, which has annualized sales of ~$1 billion ($232 million global sales in 3Q23) and the lung cancer indication underperforming skin cancer. An extension of the THIO clinical supply agreement (or other strategic agreement) would be a drop in the ocean for Regeneron, especially as it looks to provide a boost to Libtayo.
(Regeneron also has a strategic partnership with BioNTech for Libtayo in combination with BNT116, a cancer vaccine, in NSCLC).
NSCLC and Other Indications
NSCLC is the largest cancer type globally (by sales), with about 140,000 NSCL patients in the US, around 50% of which have an addressable genetic mutation such as EGFR or KRAS. THIO is being advanced in non-genetic patients, for which available treatments deliver suboptimal benefit. Establishing the efficacy of existing treatments in NSCLC is helpful in establishing a benchmark to evaluate THIO’s ongoing data.
In general, standard first-line therapy in non-genetic NSCLC is an ICI (usually Keytruda), either alone or in combination with chemotherapy for patients with PD-L1 expression. Patients with no PD-L1 expression usually receive doublet chemotherapy regimens that include a platinum-based (e.g. carboplatin, cisplatin) and other chemotherapy (e.g. pemetrexed, gemcitabine).
Keytruda’s PEMBREIZH trial in first-line NSCLC patients expressing 50%+ PD-L1 (i.e. Keytruda-sensitive patients) reported a 57% overall response rate (ORR; 30%+ tumor shrinkage), which was comprised almost exclusively of partial responses (PRs; 30% tumor shrinkage), with just 2% of patients achieving complete response (CR; 100% tumor shrinkage). Disease control rate (DCR; includes CR, PR, and SD) was 71%.
Almost all patients progress on first-line ICI therapy. The median progression-free survival (mPFS) on Keytruda is in the 6-10 month range, depending on the trial and patient demographic, and duration of response (DoR) for responders is about 8-11 months.
Second-Line
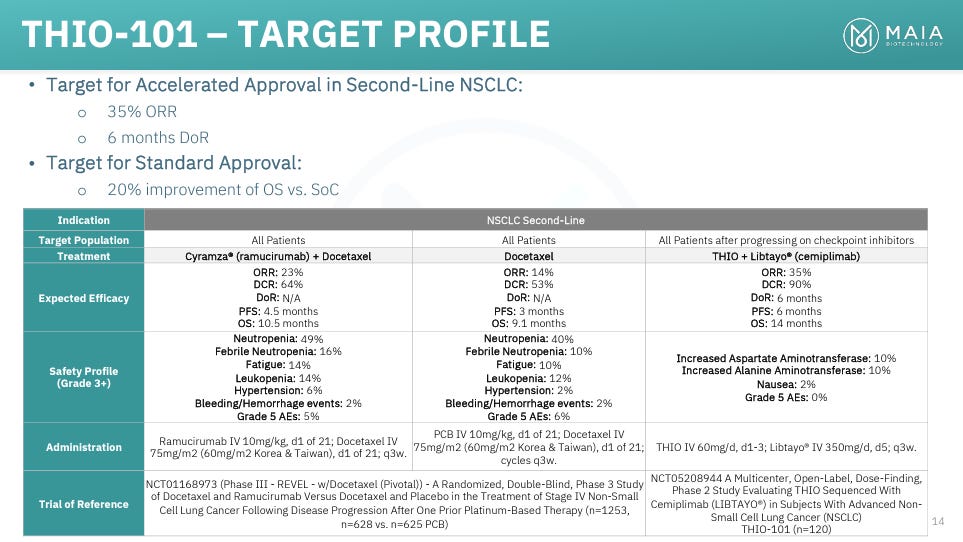
THIO is currently being tested in second- and third-line patients, where there is no clear standard of care. In second-line, most options include a cocktail of chemo and other therapies. One of the most popular and effective options is a combination of docetaxel (chemotherapy) and Cyramza (ramucirumab; VEGF antagonist). MAIA summarizes docetaxel + Cyramza efficacy in its slide deck (shown below). In its large REVEL study, docetaxel + Cyramza delivered a DCR of 64%, an ORR of 23%, and a mPFS of just 4.5 months.
Third-Line
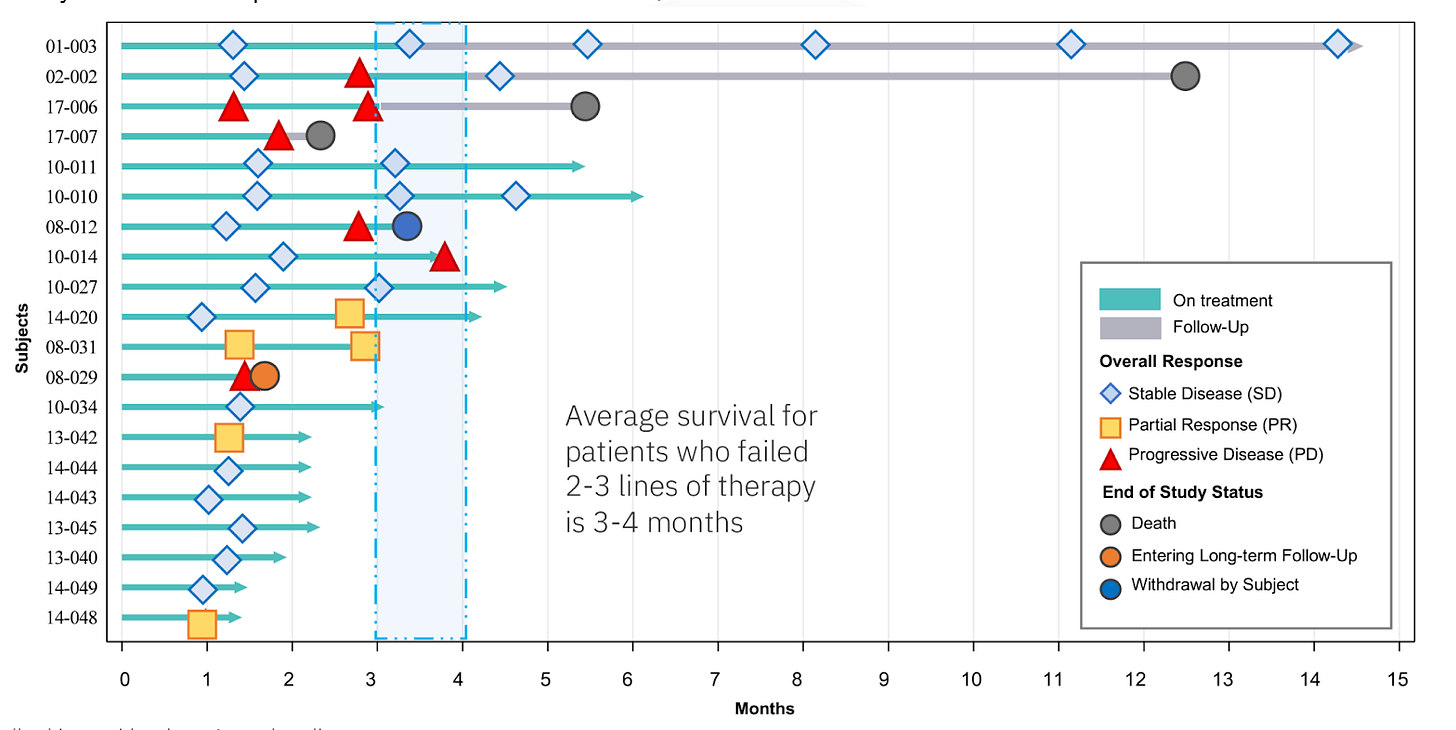
Third-line patients have particularly poor outlooks. Treatment options, again, include various combinations of chemotherapies and off-label drugs. This study of various third-line chemotherapies reported a DCR of about 36%, with very few PRs (6%) and no CRs. MAIA states that real-world DCRs in third-line NSCLC are 25-35%, with minimal PRs. Median overall survival in the above trial was 5.8 months, while MAIA states that average overall survival for third- and fourth-line patients is just 3-4 months.
Target for Approvable Efficacy
Based on the significant clinical need, MAIA is pursuing accelerated approval in NSCLC, and has set its own target ORR and DoR that it believes would be sufficient to apply. The company is primarily targeting second-line therapy, in which it is targeting a 35% ORR, which would represent a ~50% improvement over docetaxel + Cyramza, and a 6-month DoR.
While it appears based on early interim data and management’s commentary that THIO will have a good chance to hit their ORR target in second-line, MAIA has also discussed the possibility of targeting DCR as an accelerated approval endpoint. DCRs in second-line SoC are 53-64%, which MAIA is currently clearing with a DCR of 100% (as shown below), though it is unclear how much of a relative improvement THIO would need to show in order to pursue accelerated approval.
MAIA may also be able to pursue accelerated approval based on ORR and/or DCR in third-line patients. As discussed below, third-line may end up being much easier for THIO to demonstrate superiority over current treatment options, though the initial addressable patient population would be reduced.
THIO-101 Results to Date
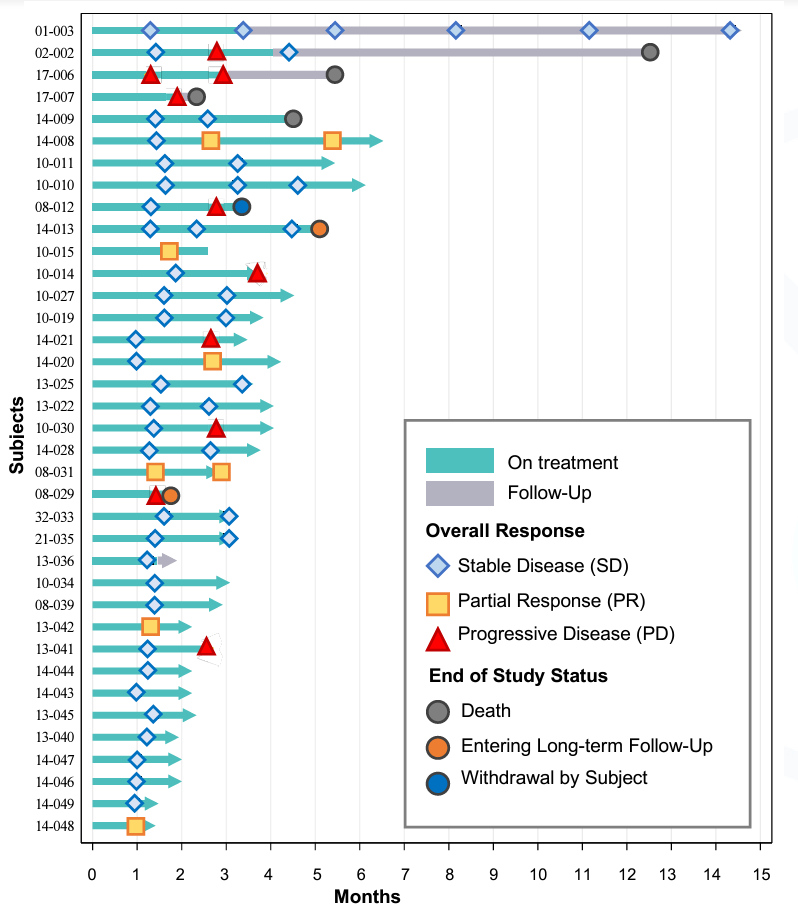
THIO’s most recent data update was given at ESMO in late October, with a data cutoff of 9/26/23. The study includes both second-line as well as third-line and fourth-line (salvage-stage) patients, all of which have failed an ICI (Keytruda in many cases). Patients in THIO-101 receive tumor scans to monitor disease progression/regression every 6 weeks. MAIA had enrolled 49 patients as of the data cutoff, with 37 patients having been evaluated with at least one follow-up scan. While the data is still early-stage and requires some context and interpretation, the preliminary signs are encouraging for THIO:
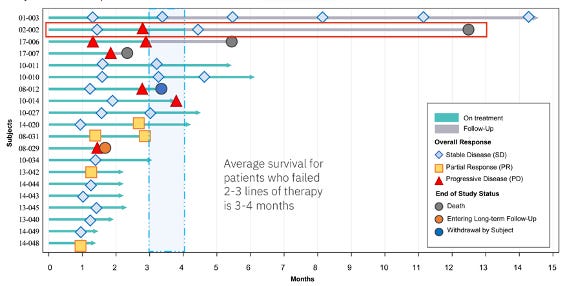
33 of the 37 reported patients across all lines of therapy remain alive as of the most recent update (more info on the deaths in the lines of therapy breakdown below). Impressively, the first 2 pilot patients that were enrolled in Australia in mid-2022, both of which were third-line, were able to survive 12.5 months (recently deceased) and 14 months (ongoing survival). The rest of the patients were enrolled in Europe over the course of 2023 and were all 1-6 months into treatment as of the data cutoff.
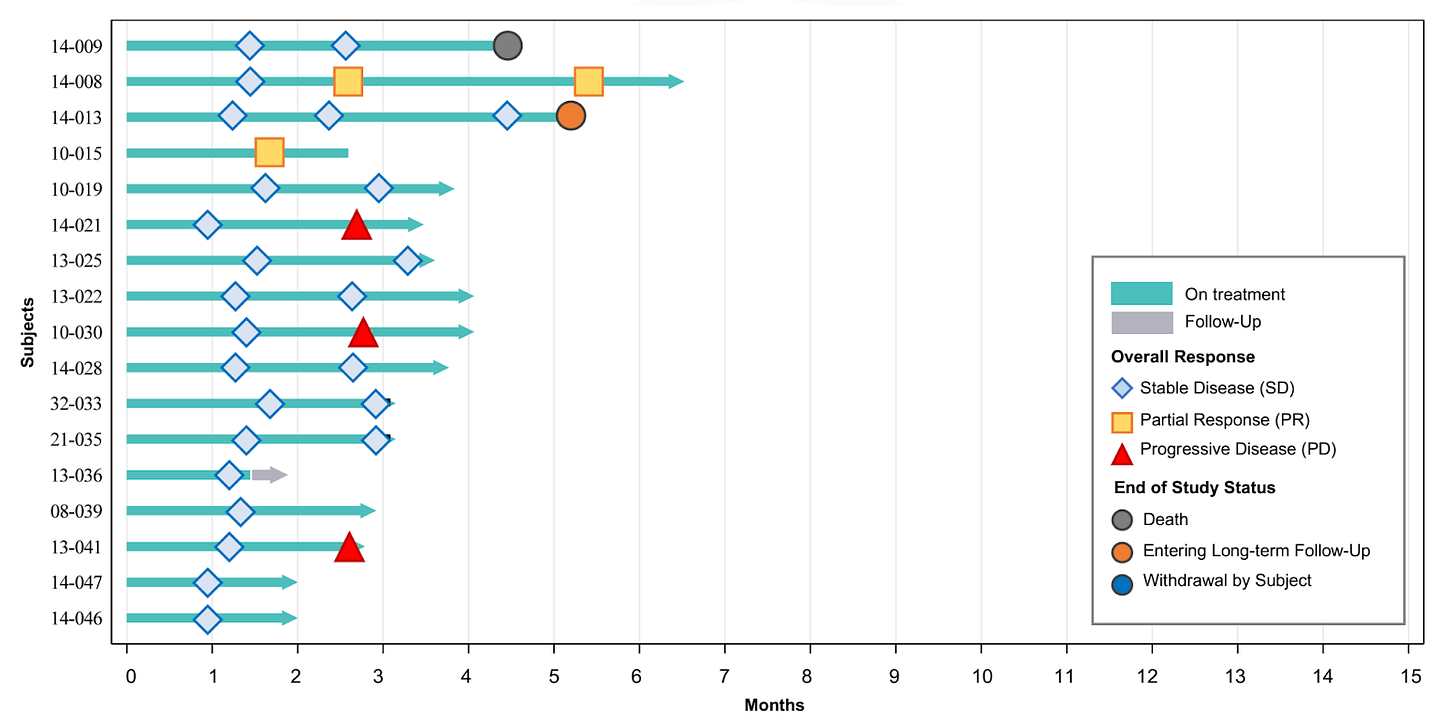
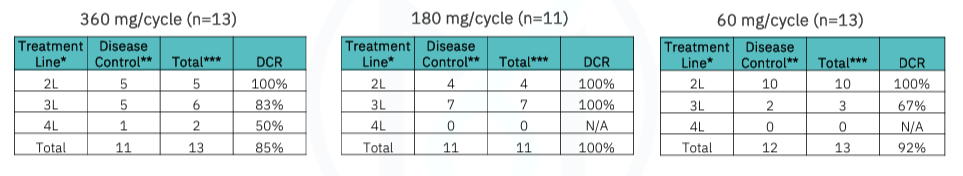
For the total treatment group, DCR was 92%, which broke down to 100% and 88% for second-line and third-line+ (third/fourth line) patients, respectively. While the company’s press release touts these topline DCRs, more important is whether patients are maintaining their disease control status. As of the data cutoff, we can see that 27 of the 37 patients (73%) were experiencing ongoing disease control. MAIA does not appear to count Patient 13-036, who withdrew from the study, leading them to state that 26 of 37 patients (70%) were experiencing ongoing disease control as of the cutoff.
As for responses, the data appear somewhat disappointing at first blush, with THIO reporting just 6 PRs (15%) and no CRs (0%). However, the data presented at ESMO is very early stage, with just a ~3-month median duration of treatment, and CEO Dr. Vlad Vitoc indicated at the Noble Capital Markets conference in early December that the 180 mg dose is now achieving a 35%+ ORR.
Second-Line
In second-line, there has been only 1 death (out of 17 patients), which the company states in their slide deck was a myocardial infarction (without cancer progression).
While initial DCR was 100%, three patients unfortunately experienced disease progression in their most recent scan. As shown in the third-line+ data below, however, it is not unprecedented for a patient to progress and subsequently regain stable disease status. PRs were achievedin just 2 (12%) second-line patients as of the data cutoff.
Third-Line
Of the 20 third-line+ patients, 3 have died thus far. It appears that the second patient (02-002) died without disease progression, though it is hard to say for sure because it appears they stopped receiving regular follow-up scans. Nonetheless, the patient lived almost 13 months as a late-stage NSCLC patient. The other 2 deceased patients failed to show any initial response or disease control, with one (17-007) dying only about two months after treatment initiation. It is possible that this patient was 1 of the 2 fourth-line patients enrolled in THIO-101.
In all, third-line patients reported an impressive DCR of 88%. Interestingly, there were four PRs (20%), meaning that third-line+ patients contributed two-thirds of the partial responses seen in THIO-101 to date. THIO’s 20% PR rate in third-line is approaching the 23% level set by docetaxel + Cyramza in second-line patients.
180 mg Dose
The company recently announced it had selected 180 mg as the dose it will attempt to advance to accelerated approval. Dr. Vitoc’s update that the 180 mg group was achieving 35%+ ORR (as of early December 2023) has interesting implications. It could mean that either 4 (or more) of the 6 patients that achieved a PR as of the data cutoff were from the 180 mg dosage cohort. Alternatively, it could mean that existing patients either flipped to PR and/or new patients not included in the September data cutoff achieved PR early in treatment, both of which would be very encouraging.
The company also gives a breakdown of disease control by dose, with the 180 mg yet to not achieve initial disease control. We would be interested to know how many of these patients, if any, subsequently progressed.
The company will now go on to enroll 100-120 total patients with the 180 mg dose.
Further Interpretation
THIO is in the very early stages of putting together an differentiated efficacy profile. Some interesting ways to consider the data:
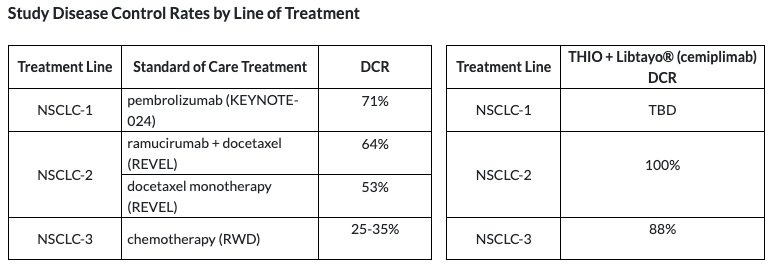
THIO’s 100%/70% initial/ongoing DCR in second-line ICI-failed patients compares favorably with Keytruda’s 71% DCR in first-line in the PEMBREIZH trial. Duration will be key here, but it is impressive to see THIO’s ability to essentially re-sensitize patients who became resistant to ICI. Interesting to consider the efficacy that THIO + ICI would deliver in first-line therapy.
THIO’s 100% DCR in second-line as of the data cutoff is also significantly higher than the 53-64% delivered by current SoC.
THIO’s 88% initial DCR in third-line patients is also more than double the 25-35% reported for SoC third-line chemotherapy regimens.
THIO’s 20% ORR in third-line is currently far beyond the single-digit ORRs delivered by third-line SoC, and is approaching the 23% seen in second-line docetaxel + Cyramza therapy.
The 180 mg dose had a 100% disease control rate as of the data cutoff.
The 35%+ ORR guidance by Dr. Vitoc for the 180 mg dose would be significantly better than SoC in both second- and third-line.
The lead two patients' survival of 12 and 14 months (ongoing) is highly unusual, much longer than the 3-6 month median OS.
Potential for Delayed Responses
At the Noble conference, Dr. Vitoc also told the story of the first patient enrolled in THIO-101 (Patient 01-003; enrolled in July 2022) who is still alive with stable disease. He shared that the patient had four cycles of THIO + Libtayo and achieved stable disease with slight tumor shrinkage. Then, in the last two scans (which switch to every three months once treatment ends), she reported 28% tumor shrinkage from baseline (just shy of a PR), showing the potential of THIO + Libtayo to have a growing effect even many months after treatment cessation.
We think it is likely that this patient continues to survive based on their improving response, which means they would be 17-18 months survived as of the next data cutoff. This patient serves as a flagship example of THIO + Libtayo’s potential and is inspiring for NSCLC patients.
There is other evidence of THIO + Libtayo’s delayed effect, including that 2 of the 6 PRs as of the data cutoff achieved their response on the second scan after initially reporting disease control. Similarly, third-line Patient 02-002 (second patient enrolled) initially achieved stable disease, then experienced disease progression, then achieved stable disease once again on their third scan, and then went on to live over a year before passing away, demonstrating THIO’s potential to activate and sensitize the immune system.
These examples may be partially explained by a known phenomenon that occurs with ICIs whereby some patients experience an initial increase in tumor size (known as “pseudoprogression”) and then subsequent response/disease control. These responses can occur even beyond six months of treatment (Jia, et al., 2019). Additionally, Libtayo’s half-life is about 21-22 days, meaning it doesn’t reach peak concentrations until the second or third dose.
Looking Forward
While the early data looks promising, THIO is approaching a critical period where it can establish and solidify its impressive in-human clinical profile over the coming weeks and months. As of this December 5th Noble conference, Dr. Vitoc said MAIA had dosed 65 patients. The next data update, which may come at the JP Morgan Healthcare conference next week, will likely add about 3 months in treatment duration for existing patients and 15-20 new patients with at least one post-baseline scan.
Some critical questions that will be answered in the immediate- and short-term:
Are patients showing improving/deepening responses at their second and especially third scan (3-5 months post-treatment)? Only 7 of the 37 patients had had a third scan as of the most recent data cutoff.
Are any of the recently-progressed patients regaining stable disease? More evidence of the resilience and delayed response effect of THIO + Libtayo would be encouraging.
What does survival look like? Are third-line patients living past the 6-month mark? When will we get an early idea of mPFS in each line of treatment? This will begin to take shape towards the middle of the year when MAIA presents at ASCO.
Will flagship Patient 001-003 continue to survive ~17 months after treatment initiation and ~13 months post treatment cessation at the expected JPM data update, and, in an upside scenario, perhaps even show a deepening to PR?
If some of these outstanding questions regarding THIO’s strength and durability of response swing in the positive direction, THIO could really begin to gain notoriety and as a foundationally different approach to oncology.
Regulatory Path
DCR vs ORR
While we believe second-line ORR as an accelerated approval endpoint is still very much achievable, esepcailly in light of Dr. Vitoc’s comments, the company already has clear separation on DCR in both second- and third-line. While not as commonly cited as ORR, DCR is an important metric in oncology that has been established as a strong predictor of overall survival (Lara, et al., 2015; Nakashima, et al., 2016). As a primary example, neither of THIO-101’s longest-lived patients achieved a partial response. THIO simply halted the progression of the disease, allowing third-line NSCLC patients to live an additional 12+ months and beyond, versus an expected overall survival of 3-6 months.
Second-Line vs. Third-Line
The company will also likely have optionality when it comes to which line of therapy it will pursue for potential accelerated approval. THIO is currently beating second-line (and first-line) SoC therapy on DCR, and is also currently beating third-line SoC on both ORR and DCR. If the current data holds (or perhaps improves slightly), MAIA will have multiple options on which it can pursue accelerated approval.
It may be that THIO’s unique mechanism delivers similar efficacy in second- and third-line treatment, which would mean the relative benefit versus SoC is more readily apparent in third-line.
Regular Approval
If THIO’s data end up being insufficient for accelerated approval, the company can still pursue the traditional approval path, which the company believes would require just a 20% improvement over current SoC to be approvable. This leaves room for a significant drawdown in the response and disease control rates, which unfortunately would make THIO a much less impactful oncology treatment, but would still allow for the approval THIO as a unique treatment option.
Investigator-Initiated Trials
Given THIO’s preclinical and early clinical-stage data, especially if upcoming readouts continue to be strong, MAIA could receive interest from academic and medical institutions in running trials with THIO. Investigator-initiated trials could potentially bolster THIO’s eventual approval package.
“Homerun” Scenario and Accelerated Approval
The company will have a very robust data set particularly by mid-2024 at ASCO and in the 2H24, with 100+ patients treated with the selected 180 mg dose with a median follow up of 6+ months. If the thesis that THIO + Libtayo responses show durability or even deepening over time, we could see a scenario where there is a significant boom of investor, scientific, and commercial interest in MAIA and THIO in 2024.
Depending on THIO’s efficacy, MAIA could file for accelerated approval in 2025. The FDA is already quite aware of THIO’s preclinical data as demonstrated by its granting of three ODDs.
Pipeline
MAIA also intends to initiate a basket trial in colorectal and other solid tumors, though it will have to wait until significant value has been created by THIO-101 to alleviate capital restraints. MAIA also has a pipeline of more potent telomere-targeting agents similar THIO that it plans to advance in the future.
Partnerships and Funding
As THIO-101 continues to report data maturing from 50-60+ patients in the coming weeks and months, we expect an increased probability of a strategic announcement, either with Regeneron or other big pharma oncology players. Potential partners include Roche/Genentech’s Tecentriq, as well as Merck’s Keytruda, both of which have been tested in combination with THIO in preclinical models. The orphan drug designated programs might be prime candidates for a strategic partnership.
An extension of the Regeneron clinical supply agreement would take a significant amount of the near-term funding burden off of MAIA. MAIA is currently burning around $4 million per quarter and had about $11 million of cash at the end of the 3Q23, pro forma for the $4 million November raise and ATM shares.
Valuation
At a $100k annual price tag for THIO, just 10% penetration of the 140k US NSCLC market would yield $1.4 billion of sales. Obviously, additional indications and geographic regions would only expand MAIA’s potential peak sales figure. As a rough relative valuation, Mirati, which is in Phase 3 targeting KRAS-mutated NSCLC cancer patients, was purchased by Bristol-Myers Squibb in October 2023 for $4.8 billion up front.
Essentially any traction for THIO and MAIA, even just to a $200 million micro-cap status, would represent a 5-10x opportunity. In the ultimate upside scenario, MAIA is a broad-spectrum oncology therapy with the potential to make a significant impact on the entire solid tumor treatment landscape, which is a multi-billion dollar opportunity.
Risks
Patients 3, 4, and 5 died within 6, 3, and 5 months of treatment, respectively. These were third-line patients, but death is potential risk for the recently-enrolled (as of the data cutoff) patients that will have their longer-term data reported in the coming week(s).
The investment thesis for MAIA relies on a durability and potential deepening of responses as the data matures, as well as increasing overall survival. Data may not deepen over time as expected.
Data so far has shown a significant deviation from the preclinical mouse data, which showed complete response rates of 60%+. While mice data rarely translates directly to humans, management’s expectation for THIO’s in-human data appear to have been somewhat tempered so far.
Relatedly, MAIA seems to have increased the number of THIO + Libtayo cycles that patients are receiving from the original study plan. Originally, patients would receive 3-4 cycles, while now patients are being dosed for much longer. While management is understandably attempting to find the best regimen THIO, it also illustrates the disconnect with THIO’s preclinical studies. We will note that THIO was used in the equivalent of first-line therapy in preclinical studies, which makes direct comparison difficult.
It is currently unclear if the company will be able to achieve a high enough ORR to pursue accelerated approval. DCR has not historically been used as a primary accelerated approval endpoint in oncology, and the standard approval pathway will push commercial timelines out significantly.
THIO-101 is currently enrolling patients exclusively in the EU. MAIA received FDA authorization to enroll US patients in October, but still has no US sites registered on clinicaltrials.gov because US patients are significantly more expensive to enroll. The FDA may require MAIA to enroll US patients, which and could be a drain on its limited financial position.
Chief Medical Officer Dr. Mihail Obrocea and Chief Financial Officer Joseph McGuire were fired in November, apparently in an effort to streamline operations, though other factors may have played a role.
THIO was trialed in the 1970’s but abandoned. It was mostly tested in hematological cancers, but did have a study in refractory colorectal patients that reported only 9% PR rate.
Conclusion
THIO’s data readouts over the next ~6 months represent a critical period for THIO’s clinical profile and a potential inflection point for MAIA if THIO begins to solidify its differentiation over second- and/or third-line standard of care. Unlike many oncology biotech companies (which focus on specific genetic mutations), however, strong results in NSCLC would portend positively for THIO’s anti-cancer activity in nearly all solid tumor types. MAIA is rather unique in the oncology space as a company advancing a fundamentally new addition to the oncology pharmacopeia. The emergence of a durable and potentially deepening efficacy signal for THIO in 2024 would represent a significant advancement for cancer patients and physicians.