INmune Bio: Imminent Phase 2 Alzheimer's Readout of Unique Asset Presents Interesting Speculative Opportunity
Strong preclinical, supporting, and early clinical data make INmune Bio's Phase 2 readout of XPro1595 in Alzheimer's disease a high risk/reward opportunity.
Thesis
INmune Bio is a very high risk/reward speculative mid-stage biotech ahead of its imminent Phase 2 Alzheimer’s data readout slated to be released this week or next. The company’s lead asset, XPro1595, brings a highly unique mechanism of action as a “selective” TNF-alpha inhibitor, which we believe along with extensive preclinical, mechanistic, and encouraging (though very incomplete) Phase 1b data, make the Phase 2 readout perhaps the most intriguing mid-stage Alzheimer’s readout opportunity that we have seen.
XPro1595 selectively targets and inhibits soluble TNF (sTNF), which has been widely demonstrated to be the inflammation- and damage-inducing form of TNF-alpha, while sparing the other form of TNF-alpha, called transmembrane TNF (tmTNF), which, interestingly, has been widely demonstrated to be anti-inflammatory and reparative in many biological contexts.
There are a number of reasons to be optimistic about the Phase 2 trial readout:
TNF inhibitors like Humira and Enbrel are amongst the best-selling blockbuster drugs of all time and proved to be broadly applicable because of their TNF-targeting mechanism (approved for 10+ inflammatory conditions).
TNF-alpha is perhaps the most central upstream mediator of the inflammatory cascade. It is also one of the most evolutionarily conserved inflammatory mediators, meaning animal studies may be more translatable to humans.
sTNF, which XPro1595 selectively inhibits, has been demonstrated to be a primary driver of inflammation and tissue damage in numerous diseases, while tmTNF, which XPro1595 spares, is shown to exert anti-inflammatory and reparative effects. There are hundreds of published studies on sTNF/tmTNF biology and XPro1595.
Approved TNF inhibitors, despite causing demyelination in some people, have been shown to impart dramatically reduced risk of Alzheimer’s disease.
A small trial of Enbrel in Alzheimer’s in 2015 showed encouraging results that likely would have reached statistical significance if tested in a larger trial.
A doctor utilizing perispinal injection of Enbrel has published numerous case studies and posted numerous videos of striking recoveries in Alzheimer’s, Parkinson’s, post-stroke, and other neurological conditions.
The XPro1595 molecule, on top of being the first selective sTNF inhibitor, is about one-sixth the size of Enbrel and Humira (~27 kDa vs. ~150 kDa), meaning it is much more capable of penetrating the brain/CNS.
XPro1595’s small Phase 1b trial reported consistently positive results across inflammatory cytokines, proteins, and imaging tests.
Two patients from the Phase 1b had dramatic responses, allowing them to regain their memory, cognition, and interest in life. Both patients remain stable 3+ years later after being enrolled in an expanded access program.
The Phase 2 trial, called MINDful, excluded patients that did not have at least one inflammatory biomarker, potentially improving the chances that patients benefit from XPro1595’s mechanism and increasing the likelihood that the placebo group declines as expected.
If the placebo group declines similarly to those in the large Phase 3 trials of lecanemab (Lequembi) and donanemab (Kisunla), about 0.6 points on the CDR-SB scale over 6 months, XPro1595 should reach statistical significance if it can deliver anywhere from stable cognition to about 0.3 points of decline on CDR-SB, subject to assumptions discussed in Handicapping Odds of Success below.
While MINDful’s primary endpoint is EMACC, a little-known cognitive assessment specifically designed for early Alzheimer’s patients (and thus more applicable to the MINDful trial than oft-used assessments like CDR-SB and ADAS-Cog), we believe the data will need to show positive and congruent results on both EMACC and the well known CDR-SB in order to get full credit from the market and a corresponding stock price movement.
That said, even in the event of an overall positive readout, investors will be looking for any inconsistencies given how difficult it has been to show positive disease modifying results in Alzheimer’s, and the market’s reaction to any of the possible outcome permutations is difficult to predict. This is where the fact that the trial is relatively short, that it enrolled in exclusively ex-US sites, and that INmune will likely look to do a capital raise in the next couple quarters, could play a significant role (amongst some other caveats discussed).
Thus, while we firmly believe inflammation-targeting therapies (likely in multi-drug combinations) will become the standard of care in Alzheimer’s over time and that XPro1595 represents perhaps the best opportunity for a therapy to break into that conversation, a position in INmune is inherently speculative.
If XPro1595 and INmune do manage to pull off the seemingly impossible, though, the implications for XPro1595, in not only Alzheimer’s but all of the various other neurodegenerative and broadly inflammatory conditions for which its mechanism has been validated in preclinical models, becomes a massive opportunity, especially given that the company is currently virtually unknown to investors.
This article focuses on 1) the scientific basis of TNF-alpha in numerous diseases, 2) the demonstrable opposing effects of sTNF and tmTNF, 3) the preclinical and Phase 1b data for XPro1595, 4) the Phase 2 trial design, and 5) the conditions under which XPro1595 can reach statistical significance.
TNF Primer
TNF-alpha is one of, if not the, strongest and most central immunomodulatory cytokines in the human body. Its ability to initiate and coordinate inflammatory cascades has led to its being labeled as a “master regulator” of the immune system. To our knowledge, there is essentially no cytokine more central to the inflammatory response (notable mentions would be IL-1b, IL-6, etc., all of which have strong associations with TNF-alpha and are lowered by TNF inhibitors). For this reason, TNF became a target of interest starting in the late 1980s and early 1990s for the treatment of inflammatory conditions, which led to the discovery of blockbuster Humira (adalimumab), along with other TNF inhibitors Enbrel (etanercept), Remicade (infliximab), and others.
Humira’s strong efficacy across various inflammatory/immunological indications led to its approval in at least 10 inflammatory indications ranging from rheumatoid arthritis to ulcerative colitis, reaching peak sales of $21+ billion in 2022 (before biosimilars became available in early 2023), making it among the two or three most successful drugs of all time.
Interestingly, after anti-TNF therapies became widely prescribed, scientists began to publish papers showing that while patients with rheumatoid arthritis were nearly 8x more likely to develop Alzheimer’s than the general population, patients taking TNF inhibitors had about a 60% lower risk of Alzheimer’s than untreated patients, bringing their overall risk for Alzheimer’s in line with, or, according to some studies, below that of the general population.
While meta analyses vary from 2-8x increased risk of Alzheimer’s amongst RA and other inflammatory disease patients, and the risk reduction affected by TNF inhibitors ranges from 30-70% depending on the drug and patient population, the signal is consistent across analyses—TNF inhibition lowers Alzheimer’s risk, at least in those with aberrant underlying inflammation.
These observations led researchers to trial Enbrel in a small Alzheimer’s trial, which, though it did not (and was not designed to) reach statistical significance, showed some very interesting and encouraging data that we discuss below. While we think Enbrel likely would have reached statistical significance in larger trials, Pfizer and Amgen both elected not to pursue Enbrel in Alzheimer’s, primarily due to its inability to cross the blood-brain barrier, as well as upcoming patent expiration and other strategic initiatives.
One of the most interesting twists in the story of TNF inhibitors, though, is that despite being extremely efficacious in numerous inflammatory conditions and lowering the risk of Alzheimer’s, cardiovascular events, and other off-label conditions, they have also been found to increase the risk of infectious disease, some forms of cancer, demyelination (think Multiple Sclerosis), and, paradoxically, some autoimmune conditions.
The explanation for this dichotomy primarily lies in the fact that there are two different forms of TNF—soluble TNF (sTNF) and transmembrane TNF (tmTNF)—with dramatically different effects in the body.
Soluble TNF vs. Transmembrane TNF
Researchers have shown through dozens (likely hundreds) of publications that tmTNF, which is TNF-alpha embedded within cell membranes, is actually primarily anti-inflammatory and regenerative, while sTNF, which is TNF-alpha cleaved from the cell membrane by enzymes and floating freely in between cells, is pro-inflammatory and the major driver of TNF-mediated diseases.
Simplifying a complex system, the different physiological effects of sTNF and tmTNF can be distilled down to the fact that sTNF primarily binds to the TNFR1 receptor (which has shown to be pro-inflammatory, etc.), while tmTNF has been shown to primarily bind to TNFR2 (which has shown to be anti-inflammatory, reparative, etc.). This explanation leaves out some nuance, but is accurate for the purposes of understanding INmune and XPro1595. The following diagram provides a comprehensive overview of the opposing actions of the two receptors.
Interestingly, while TNFR1 receptors are expressed essentially ubiquitously on almost all cell types, TNFR2 receptors are only expressed on a handful of specialized cell types, including endothelial cells and certain immune cells like fibroblasts and Treg cells.
Starting all the way back in the 1990s, and even still to this day in 2025, researchers have been publishing on the deleterious effects of sTNF and TNFR1 across nearly every body system and disease type.
Research on Opposing Effects of TNFR1 and TNFR2
Neurodegeneration/Neuroprotection
Studies have shown that sTNF and TNFR1 are amongst the core divers of multiple neurodegenerative diseases, including Alzheimer’s, Parkinson’s, ALS, and MS, as well as stroke-related damages, at least in animal models.
Multiple Sclerosis and Demyelination
TNFR2 blockade has been found to cause demyelination and impairment of remyelination processes in the brain, which likely underlies the increased risk of developing MS for patients taking non-selective TNF inhibitors like Humira, while TNFR2 agonism has been shown to promote remyelination.
INmune points out that this dynamic leads to etanercept and XPro1595 exerting opposite effects on myelination.
Cardiovascular
TNFR1 has been shown to be involved in heart inflammation and worsening function in heart failure and other cardiovascular diseases.
This list leaves out dozens of studies, including those in models of sepsis, liver disease, diabetic kidney disease, COPD, åsthma, inflammatory skin conditions, and more.
Attempts At Selective TNF Targeting
Given all of the disease implications, there have been numerous attempts over the past 10+ years to develop various treatment modalities, including TNFR1 antagonists, TNFR2 agonists, TNF converting enzyme (TACE) inhibitors. This is still a very active area of drug discovery, with research being published as recently and frequently as June 2025, May 2025, March 2025, and January 2025.
Therapeutic potential of TNFR2 agonists: a mechanistic perspective
Tumor necrosis factor receptor-2 (TNFR2): an overview of an emerging drug target
The TNFR1 Antagonist Atrosimab Is Therapeutic in Mouse Models of Acute and Chronic Inflammation
Exploring TNFR1: from discovery to targeted therapy development
A TNFR2-Specific TNF Fusion Protein With Improved In Vivo Activity
TNF-α signaling: TACE inhibition to put out the burning heart
Sanofi small molecule TNFR1 inhibitor balinatunfib recently came up short of statistical significance in a psoriasis trial, though the company is still running trials in ulcerative colitis, Crohn’s disease, and rheumatoid arthritis. Private biotech TRexBio, which raised in $84 million last November, is currently advancing its TNFR2 agonist in a Phase 1 trial in atopic dermatitis.
T Regulatory Cells
Perhaps the key mechanism underlying the opposing biological effects of sTNF/TNFR1 and tmTNF/TNFR2 is their opposing effects on T regulatory cells (Tregs), which are known as the “brakes” on an overactive immune system. sTNF/TNFR1 signaling has been found to suppress Tregs, while tmTNF/TNFR2 signaling has been shown to stimulate expansion of Tregs.
Dysregulated Tregs have been implicated in lupus, multiple sclerosis, rheumatoid arthritis, and neurodegenerative diseases. This is the thesis that companies like Nektar Therapeutics and Coya Therapeutics are attempting to address in various diseases (via flawed mechanisms, we would argue).
Not only does TNFR1 activation inhibit Treg cells, but activation of TNFR2, which is expressed on only a few cell types, one of which is on Treg cells, actually causes Treg expansion and activation.
Role of TNF–TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications
TNFR2 promotes Treg-mediated recovery from neuropathic pain across sexes
The TNF-α/TNFR2 Pathway: Targeting a Brake to Release the Anti-tumor Immune Response
Modulation of Regulatory T Cell Activity by TNF Receptor Type II-Targeting Pharmacological Agents
While we have left out other interesting or potentially key areas of research, including TNFR1’s role in priming/triggering the NLRP3 inflammasome and TNFR2’s ability to positively regulate stem cells, this sampling of TNF-related research lays the groundwork for understanding the attractiveness of XPro1595’s mechanism. These studies also provide context into how XPro1595 could be useful in a host of diseases outside of Alzheimer’s.
XPro1595
Throughout the development and commercialization of TNF inhibitors (starting with Enbrel and Remicade approvals in 1998), groups of researchers were also honing in on the importance of sTNF, including those at pharmaceutical companies. In 2003, researchers working for Xencore discovered XPro1595 (also called DN-TNF and pegipanermin; formerly called XENP324).
XPro1595, technically referred to as dominant negative TNF (DN-TNF), is a clone of endogenous TNF, but with three amino acid point mutations. These mutations allow XPro1595 to assume the natural behavior of endogenous sTNF, which is to combine with other sTNF monomers to form an active sTNF homotrimer (three monomers bound together). XPro1595’s is incorporated into these homotrimers and prevents them from being able to bind to TNF receptors, essentially sequestering and biologically deactivating soluble TNF.
In addition to the advantages of selectively blocking sTNF, XPro1595 also has the advantage of being significantly smaller than all available TNF inhibitors. At only 27 kDa, XPro1595 is a fraction of the size of the ~150 kDa Humira, Etanercept, and Remicade molecules. This is likely why XPro1595 is able to penetrate the brain/CNS effectively, and why it may have even greater benefits in CNS disorders versus non-selective TNF inhibitors.
At the time of its discovery, Xencor’s CEO Dr. Bassil Dahiyat, who was also the lead author on the discovery paper, spoke glowingly about the potential for a selective sTNF inhibitor. However, for various reasons, XPro1595 became deprioritized, perhaps as Dr. Dahiyat handed the CEO role over to Harry Stylli for a couple years before resuming the position, or as the company’s XmAb antibody platform began to take off (signing five deals in one year circa 2005), making entering a TNF inhibitor space with behemoths Humira and Enbrel less appealing.
Still, there have been dozens (management indicates 75+) of studies conducted on XPro1595 since its discovery, by researchers affiliated with INmune (which in-licensed XPro1595 in 2016), Xencore, and third-party researchers around the world. The company’s Our Science page enumerates most of the studies, while this report from the Alzheimer’s Drug Discovery Foundation also provides a good overview. Dr. Malu Tansey, one of the original researchers on XPro1595, a leading TNF-alpha and neurodegenerative disease researcher, and frequent participant in INmune’s webinars, is also a good resource for the understanding of sTNF and XPro1595.
Select XPro1595 Papers:
Phase 1b Data
INmune began its first-in-human Phase 1b trial of XPro1595 in Australia in 2019. The trial enrolled a total of 12 patients that had at least one aberrant biomarker of inflammation (e.g. HS-CRP, HbA1c, APOE4). The analysis of the Phase 1b data focused on the highest dosing cohort, 1 mg/kg (n=6), as the lower doses had weaker responses. You can watch the company’s full webcast discussing the Phase 1b results here.
The trial sought to measure cognitive, plasma, CSF, and imaging endpoints of Alzheimer’s disease progression/improvement.
One of the key findings was a consistent reduction of the levels of 30+ cytokines in the CSF, including key cytokines associated with neurodegeneration like IL-6.
The results also showed significant improvements in the levels of key neurodegeneration-associated and neuronal integrity proteins like neurofilament light (Nfl), neurogranin, and contactin-2.
A small but important nuance we will point out is that the data for these improvements in CSF biomarkers was originally presented as log-scale changes (shown above), but was then converted into percentages (shown below), which, for technical/scientific reason aren’t completely accurate.
These changes in inflammatory and neurodegeneration-associated proteins also remain consistent with pTau-217, which has become one of the most utilized and validated biomarkers in Alzheimer’s disease, showing a significant decrease after 12 weeks of XPro1595. You can also see that the percentage reductions in pTau-217 were very consistent across each of the patients at the 1 mg/kg dose cohort.
The company also included a number of imaging studies in the Phase 1b trial in order to assess axonal integrity, neuronal inflammation, and neuronal survival. Patients in the highest dosing cohort, all of which enrolled in a 12-month extension study, had MRI scans at 3, 6, 9 , and 12 months. The results showed improvements in axonal integrity (apparent fiber density) and a decrease in white matter free water (a measure of inflammation).
As far as cognitive endpoints, the company (to our knowledge) never disclosed patients’ actual scores on MMSE and the other cognitive endpoints that were administered. While this is a bit curious and we acknowledge it in the Risk Factors section of this article, it would be reasonable to argue that separation on these tests would not be expected in a very small and short duration trial, though it still would have been interesting to see.
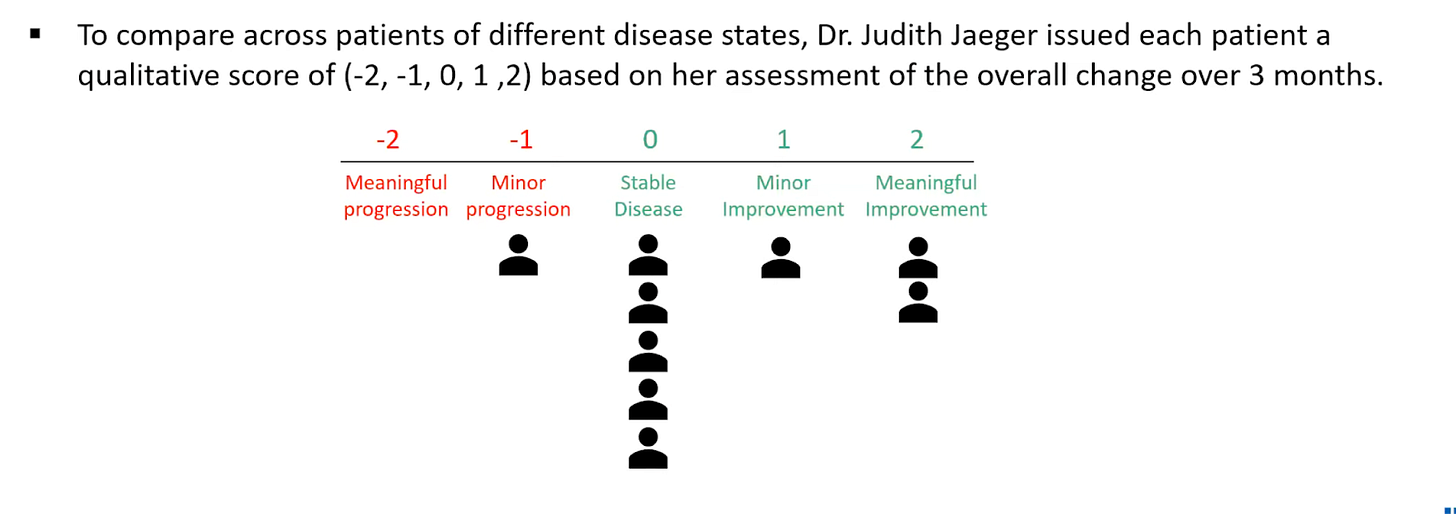
Instead, the company included a qualitative assessment by Dr. Judith Jaeger, who is the founder of CognitionMetrics, a company specializing in consulting biopharma companies on assessment and measurement of Alzheimer’s patients. INmune had Dr. Jaeger rate patients’ overall change in their condition on a scale ranging from “Meaningful Progression” to “Meaningful Improvement”. While this was based in part on patients' scores on cognitive exams, it is inherently subjective and should be taken with a grain of salt. That said, all but one patient had either stable or improved cognition after 3 months.
While there are a few important nuances, the Phase 1b data is one of the more thorough small data sets we have seen from a small neurodegenerative biotech company. In total, these data give a strong proteomic, cytokine, and imagining-based foundation for XPro1595’s potential activity in Alzheimer’s, which will be borne out further with the cognitive (and other) data from the Phase 2 readout.
Phase 1b Patient Anecdotes
The other interesting thing that came out of the Phase 1b is a couple of very stark patient anecdotes.
In the beginning of the first interim data webcast, principal investigator in the trial Dr. Rosalyn Lai shared the stories of two patients that had been barely able to communicate and care for themselves because of their Alzheimer’s. After being treated in the Phase 1b trial, Dr. Lai reports that these patients had remarkable improvements back towards their premorbid cognitive abilities, personalities, interests, and ability to care for themselves. One of these patients shared his experience on a video on INmune’s YouTube page. He reports laughing more, being able to pursue his goals and dreams again, and being more confident in his abilities overall.
After completing the full 12 months of treatment under the open label extension study, both of these patients, along with INmune and their healthcare providers, arranged to stay on treatment with XPro1595 through a compassionate use program in Australia. In April 2024, INmune provided comments from both patients’ doctors after over three years of taking XPro1595, with both indicating that their patients continued to be “stable” and/or that “cognitive decline has stopped”.
So these two patients showed rapid improvements in cognition in the first 3-6 months of treatment that was then maintained for years without significant further deterioration.
While this would be an amazing effect to show in the average patient being treated with XPro1595, based on Dr. Judith Jaeger’s assessment that the majority of the patients in the Phase 1b trial experienced stable cognition (rather than marked improvement), it is likely these stark responses will only occur in a minority of patients. We also note that both of these patients were relatively young at their age of diagnosis (59 and 61, respectively), which may have had something to do with their strong responses.
Phase 2 Trial and EMACC Endpoint
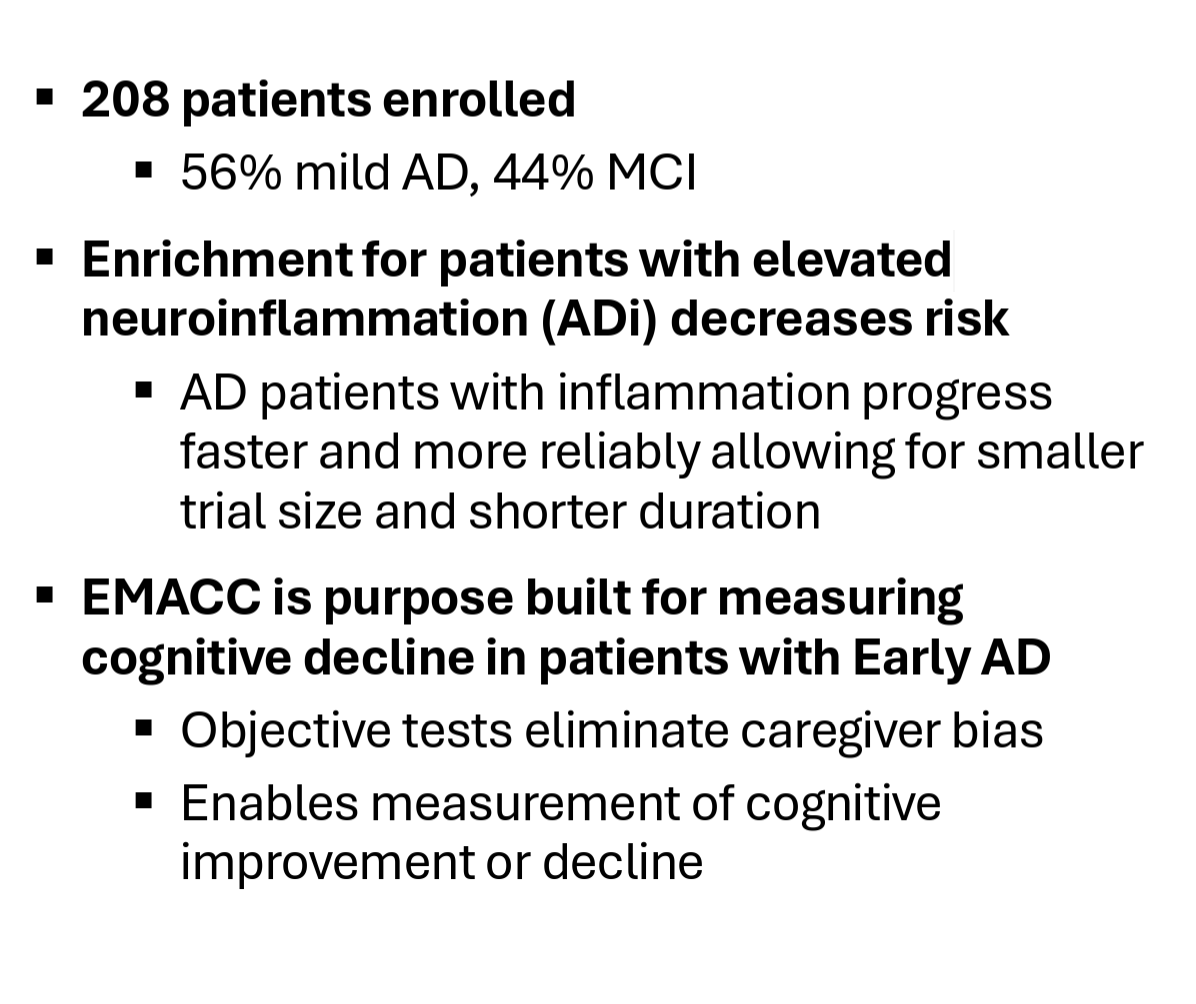
The Phase 2 MINDful trial, which completed enrollment in November 2024, enrolled 208 early Alzheimer’s patients randomized 2:1 between the treatment (1 mg/kg) and placebo groups. The trial’s inclusion criteria enriched for patients with early AD that have elevated biomarkers of neuroinflammation (i.e. hsCRP, HbA1c, ESR, or APOE4 allele).
The company shared the baseline characteristics of the trial’s participants in April, sharing that 56% of patients were mild AD patients vs. 44% MCI patients, an average age of 72 years old, that 69% of patients were APOE4 carriers (heterozygous or homozygous), and that 64% of patients met more than one inflammatory enrichment biomarker.
We believe these enrichment measures should increase the chances of XPro1595 delivering a positive effect on cognition, both because they play into XPro1595’s mechanism and because it should ensure that the placebo group declines as expected.
Changes in cognition will be measured by a number of tests, with the primary endpoint being a relatively unknown cognitive test called the Early/Mild Alzheimer’s Cognitive Composite (EMACC). This test was specially created in 2017 by Dr. Judith Jaeger at CognitionMetrics in conjunction with Lundbeck Pharma with the specific goal of creating a cognitive test that was sensitive enough to detect subtle changes in cognition in early Alzheimer’s. This webcast with Dr. Jaeger and the INmune team really goes in depth into EMACC, its creation, and its validated superiority in early Alzheimer’s patients.
The reason INmune is using EMACC as the primary endpoint rather than a more traditional test like CDR-SB (which it is still including as a secondary endpoint) is because EMACC is a performance-based test designed to measure subtle changes in cognition, whereas traditional tests like CDR-SB rely on physicians’ and caretakers’ qualitative assessment of patients symptoms, which can be difficult to detect in early disease.
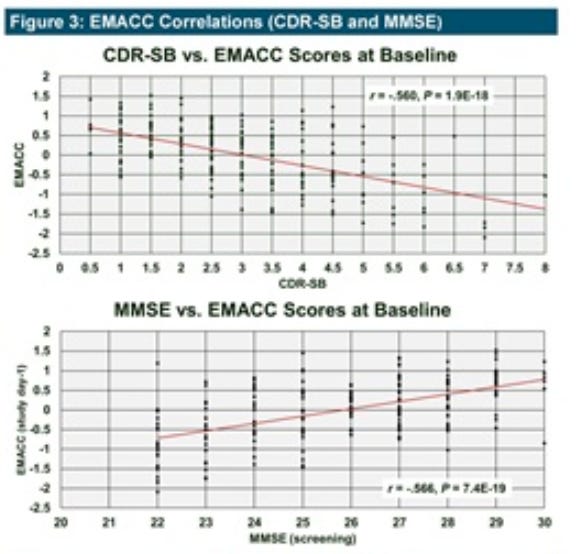
The company’s poster at AD/PD 2025 in early April also confirmed that the baseline EMACC scores of patients enrolled in the Phase 2 correlated closely with CDR-SB and MMSE, both classically used gold-standard metrics.
While our investigation of EMACC has convinced us that it is indeed the most appropriate test for INmune Phase 2 trial, we believe the company will still need to show benefit on “gold standard” metrics like CDR-SB in order to get full credit from the market.
Handicapping Odds of Success
While there may be some hope for the Phase 2 to report actual cognitive improvement from baseline, such as was seen in the two hyper-responder patients from the Phase 1, the most reasonable goal, and the stated goal of the company, is to demonstrate cognitive maintenance, that is, halting cognitive decline.
Assuming XPro1595 can deliver relatively stable cognition in the treatment group, the next question is to estimate how the placebo group will perform. The company uses Biogen/Eisai’s Phase 3 trial of lecanemab (Lequembi) as an analogue, which is a great comparison due to the very similar patient characteristics. Both trials reported 60%+ of patients carrying APOE4 and a baseline CDR-SB score of ~3 (reflecting early Alzheimer’s disease).
The placebo group in the Phase 3 lecanemab trial worsened by about 0.6 points after 6 months (roughly the duration of INmune’s Phase 2 trial), while the treatment group declined by about 0.4 points (exact results were not given for the 6 month mark).
If placebo patients decline similarly in INmune’s Phase 2 trial, INmune states that the trial is well powered to reach statistical significance. The company also believes that placebo patients in its Phase 2 trial may deteriorate faster than patients in the lecanemab trial due to the inflammatory enrichment biomarkers.
Based on the design of the MINDful trial, if the placebo group worsens by the expected 0.6 points on CDR-SB and XPro delivers stable cognition, the study will be strongly statistically significant (p < 0.001). This calculation assumes a standard deviation of 1.0 of CDR-SB for both groups, which we think is reasonable.
Regardless of where the treatment and placebo groups shake out, these assumptions suggest that if the change for the treatment group is ~0.3 points better than the placebo group, the trial will reach statistical significance.
With a more conservative (and less realistic for such a short duration trial, in our opinion) standard deviation assumption of 1.5 points on CDR-SB for both the treatment and placebo groups, the XPro1595 group would need to outperform the placebo group by 0.44 points in order to reach statistical significance.
Etanercept Trial Comparison
As mentioned earlier, a group of researchers ran a small trial of etanercept (Enbrel) in 41 Alzheimer’s patients in 2015. Even though the trial did not meet statistical significance (which was expected given its small size), in our view, the results of the trial are very encouraging for TNF inhibitors in Alzheimer’s.
Patients showed improvement versus the placebo group on essentially every cognitive and neuropsychiatric test employed, with particularly impressive results on MMSE, ADAS-cog, and NPI, where the etanercept group either essentially halted decline or declined significantly slower than the placebo group. You can also see that the trial almost reached statistical significance (p = 0.07) on MMSE, despite having only ~15 patients in each arm of the trial.
One important piece of context is that the patients in the etanercept trial were a bit more advanced in their disease state than those enrolled in INmune’s Phase 2 (baseline MMSE score of ~20 vs. ~25), which may have led to larger declines in placebo patients.
Importantly, etanercept delivered these results despite being a non-selective TNF inhibitor that does not significantly cross the blood-brain barrier due to its molecular size.
Regarding etanercept, we would also point out interesting data points from Dr. Edward Tobinick (and others), who has demonstrated rapid and marked improvement in stroke, Alzheimer’s, Parkinson’s, and other neurological conditions using perispinal injections of etanercept. This video depicts a seemingly miraculous recovery in a post-stroke victim, and there are hundreds of similar videos on the same YouTube channel.
Positive (though incomplete) data from etanercept makes us incrementally optimistic on the probabilities of XPro1595 in Alzheimer’s.
Implications of a Positive Readout
As discussed above, INmune’s Phase 2 trial would likely reach statistical significance if XPro1595 can outperform the placebo group by 0.3-0.4 points or more in terms of change from baseline on CDR-SB. We think both EMACC and CDR-SB have to either reach statistical significance or at least show very clear trends that narrowly miss statistical significance in order for the trial to really garner attention from the market and drive significant share price appreciation.
Since EMACC is significantly more sensitive than CDR-SB in early Alzheimer’s patients, it is reasonably likely that EMACC reaches statistical significance while CDR-SB does not. In this case, we think if the trend in CDR-SB is clear and all of the other supplemental data are positive, the company can still get near-full credit for the results.
Strongly and unilaterally positive results, on the other hand, would essentially validate XPro1595’s entire mechanistic rationale not only in Alzheimer’s but in other neurodegenerative and inflammatory diseases in general, which could result in a significant move for the stock, especially considering the company’s low float, ~25% short interest, and general low trading volume and lack of market awareness.
All of that said, even if the results are positive, it is very difficult to handicap how the market will react to a small ex-US trial from a small company that is in need of a capital raise.
Financial Position
The company is in a pretty tight cash situation with $19 million of cash as of 3/31/25 and an additional $2 million raised in 2Q25. The company is burning ~$7 million per quarter, which may go down slightly now that the Phase 2 trial is over, but still creates a bit of an overhang on the readout as the company will obviously be looking to do a raise. This shouldn’t be a problem if the results turn out to be great, but could be a problem if results are equivocal.
We also note that the company has about 1.55 million warrants outstanding exercisable at a weighted average price of $9.59/share (only exercisable for 30 days following the Phase 2 readout), as well as 2.34 million warrants outstanding at an exercise price of $6.40/share. If all of these warrants are exercised, the company could see an inflow of ~$30 million.
Other Considerations
It is also worth noting that INmune has two other pipeline programs, one of which, CORDstrom, is an interesting stem cell-based program being advanced in an orphan skin disease called recessive dystrophic epidermolysis bullosa (RDEB). This program came out of the woodwork in February when the company announced positive results from an investigator-initiated trial that it had been running in collaboration with Birmingham’s Children’s Hospital in the UK, along with the awarding of an Orphan Drug Designation from the FDA and the company announcing its intention to file a BLA for CORDstrom by the end of 2025. It is a complicated orphan designation and the company hasn’t given a ton of follow up information regarding the intended BLA submission, but the program presents an interesting tangential aspect to the INmune story that could both add incremental upside over the course of 2025 and 2026 and perhaps (though maybe unlikely) provide some downside protection.
Risks
Alzheimer’s Indication
The Alzheimer’s indication has obviously been perhaps the most challenging indication to develop new therapies for, especially disease modifying therapies. The historical odds are very much against INmune.
Biotech Market Conditions
Even if the readout is largely positive, any messiness or inconsistencies in the data could lead to a negative reaction from a biotech market that has become extremely demanding of mid-stage clinical results when it comes to awarding large stock price movements.
Data to Date
In general, while there is a large body of preclinical, mechanistic, and supporting data for the idea of using a selective TNF inhibitor in Alzheimer's, XPro1595 has very limited clinical data in humans to date. While the Phase 1b had a number of limitations, including its small size, it is also a bit perplexing/concerning that the company (as far as we know) never shared the results of any of the cognitive tests that were included in the Phase 1b (EMACC, CDR-SB, NPI, etc.).
The full results of the Phase 1b were never presented in a peer-reviewed scientific journal, which is a bit odd and, in addition to the above, suggests the cognitive data points may not have looked good.
Extension Trial
It appears only two of the original five (or six) patients that enrolled in the 12-month extension trial were encouraged enough by their results to continue on therapy under an expanded access program. Two of the patients that were in the 12-month extension study also appear to have dropped out after the 9 month mark. The company has not given any explanations as far as we can find.
Trial Limitations
The Phase 2 MINDful trial is only 28 weeks, which is a rather short duration to show a benefit on cognition, especially for early-stage patients for which the placebo group may decline slower than later-stage patients, and especially on the insensitive CDR-SB endpoint that most investors will be more familiar with than the EMACC primary endpoint. Some may also raise questions about why the study was conducted in ex-US countries, though we don’t think this is much of an issue.
XPro1595
This drug has been sitting around for a long time, and even though this can happen with drugs that get lost in the shuffle, it raises the question of why Xencore and other researchers would fail to advance XPro1595 into clinical trials for over a decade.
This recent Parkinson’s animal model failed to show benefit with XPro1595, although other preclinical trials have shown benefit.
Financing Overhang
The company will likely look to do a raise in the next couple quarters if the Phase 2 results are positive. The company could receive up to $30 million from warrant exercises in the event of a positive readout, but will likely look to add to that in order to support a large Phase 3 program, as well as additional indications for XPro1595.
Competition
Even if the Phase 2 readout is successful, XPro1595 will still be a few years away from commercialization. It is possible that other TNF- or inflammation-targeting drugs emerge as promising in Alzheimer’s (though we think combination therapy regimens could mitigate this risk). There are multiple ongoing efforts to develop TNFR1 inhibitors and TNFR2 agonists.
Conclusion
While a positive result in an Alzheimer’s study seems like a moonshot, we think the extensive preclinical and mechanistic research on both TNF-alpha and XPro1595’s selective TNF inhibition mechanism, as well as the encouraging small Phase 1b data set (including two patients that are apparently still stable today), present an intriguing opportunity in INmune’s imminent Phase 2 readout.




























Very good overview of the situation. There are a few details you left out that are important. First, the very clearly communicated reason for the ex-US P2 trial is due to the FDA hold that went much longer than expected. The company was eager to include US sites but for the FDA’s heel-dragging. Second, in case of a successful P2 outcome, the company’s cash position shouldn’t be any concern, as $30 million in warrants will be immediately exercised to provide close to a year of capital. And excellent results will likely lead to non-dilutive licensing deals months later. I do expect them to use their shelf to raise $100 million+, but that shouldn’t affect the stock price. Finally, given the qualifications of the neuroscientists handling the XPro data and trial design, the only reason to be skeptical of the P1b results not being fully disclosed would be that management is hiding something. BUT, they own a very large percentage of the company and have bought more shares at several recent financings, so they are competent enough to design a trial that they truly believe will succeed and have invested the money to support that. Unless they’re delusional, we have little reason to question the “missing” data. So these seemingly small details you left out provide just more evidence for why the stock is heavily shorted and the bet is so asymmetric.
There is some recency bias going on here. Three decades old AChEIs (donepezil etc) had effect size in cohen's d 0.2 to 0.25 against placebo in mild AD with ADAS-cog that has close to same properties as CDR-SB, cohen's d are interchangeable between them. That is ~ 2x larger than lecanemab against placebo in 6 months.
What is the possibility that XPro1595 yields equivalent or better results against placebo than donepezil? XPro1595 has shown large objective benefits from the baseline in dMRI (FW, RD, AFD), NfL (-84%), p-tau217 (-45%) which are three best markers longitudinally within-subjects to correlate with cognition decline (correlations are in range of 0.3 to 0.6 with cognition). One can find same type of correlations from other diseases, but has to look for CRP in rheumatoid arthritis or ProBNP in heart failure. They are that tight correlations.
EEG change in alpha waves (+0.5Hz) and power (+18%) was 2-3x larger in 4 weeks than what is seen with AChEIs at 6-12 months vs placebo. Such big alpha wave increase is typically seen only after spontaneous recovery of mild traumatic brain injury in few months (9.0->10Hz).
Diffusion MRI changes were a lot larger than for any MS drug approved for RRMS even though in MS they follow different locations than in AD and results in dMRI are not truly interchangeable between any two diseases. With XPro1595 the dMRI free water change -45% at 12 months imply that moderate AD patient has gone from avg mod AD --> healthy aging level of FW (there is naturally little FW along the white matter tracts) and this has occurred with approx the same magnitude in all subjects as the error bars are so narrow for N of 3 to 5.
I encourage you to find out how free water increase in white matter tracts leads to decrease in electrical conduction speed, how this leads to glial cells sensing that "you dont need these synapses anymore" and then they phagocytose them away from you due to activity-dependent modulation of synapses by microglia, astrocyte and neuronal pathways.