Fractyl Health: One-Time Procedure to Lock-In GLP-1 Weight Loss; High Risk, High Reward
Intro
Fractyl Health (GUTS), a little-known medical device company advancing a one-time procedure that promises to cement the weight loss gains imparted by GLP-1 drugs and allowing patients to live drug-free at a new lower metabolic set point, is gearing up for an eventful next 12 months, beginning with the results of a 45-patient study in GLP-1 discontinuation patients expected to report data in the month of September (any day now).
The device and respective procedure, named Revita, involves the hydrothermal ablation of a section of duodenal lining, a tissue which has been found to undergo changes in response to high-fat, high-sugar diets that disrupt the body’s metabolism such that it regulates to and defends a higher weight. The idea is that the post-ablation regrowth of this lining causes a resetting of the patient’s metabolic set point, potentially allowing patients to maintain their GLP-1 weight loss without staying on a drug indefinitely.
Revita is already approved in Europe as a standalone treatment for Type 2 diabetes (T2D), reporting 4-10% body weight loss and ~1.5% HbA1c reductions across 250+ patients as a standalone procedure (depending on the length of duodenal ablation), but has not really been commercialized due to the onerous requirements for achieving reimbursement in European jurisdictions, as well as, in our opinion, clear yet mediocre/uninspiring efficacy as a standalone treatment, which we also believe contributed to slow enrollment in Fractyl’s large US trial in T2D patients (the REVITALIZE trial, which was paused in January to prioritize financial resources for the GLP-1 discontinuation indication).
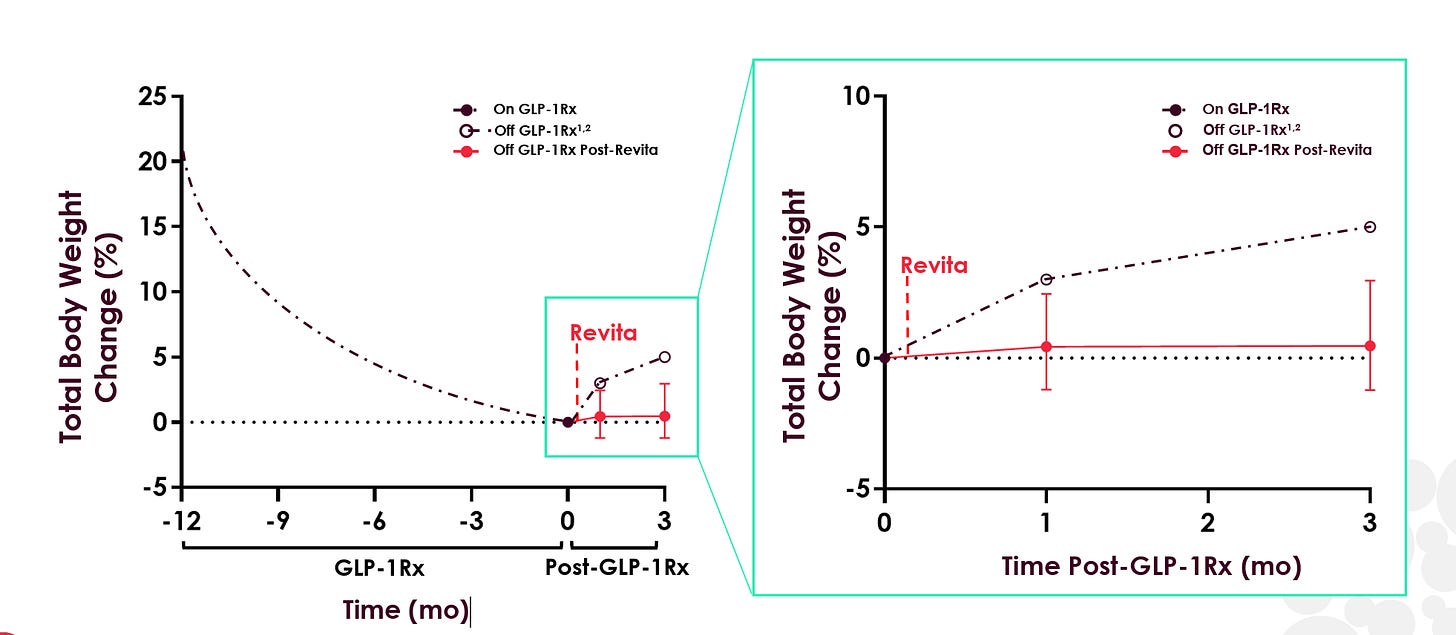
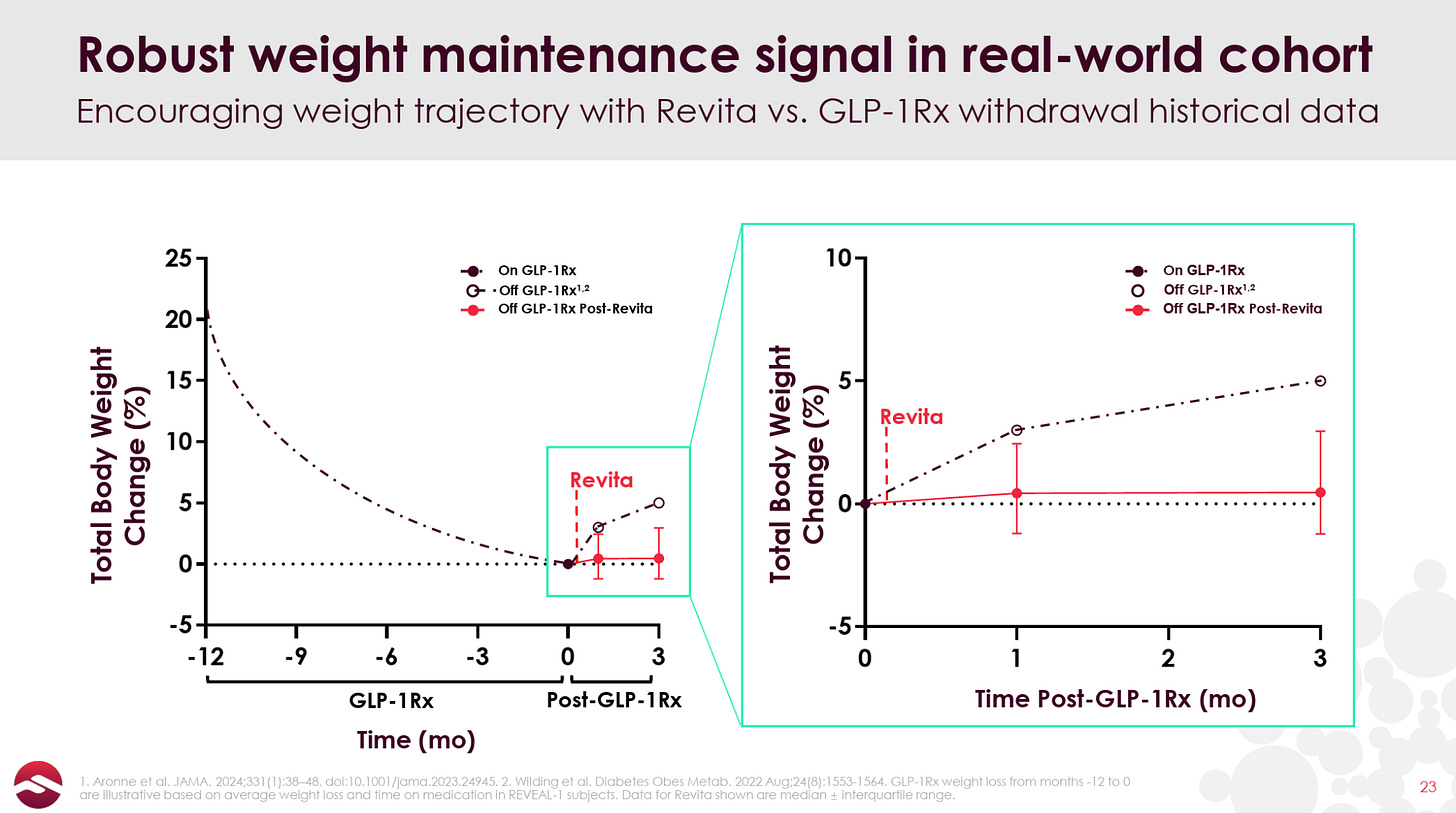
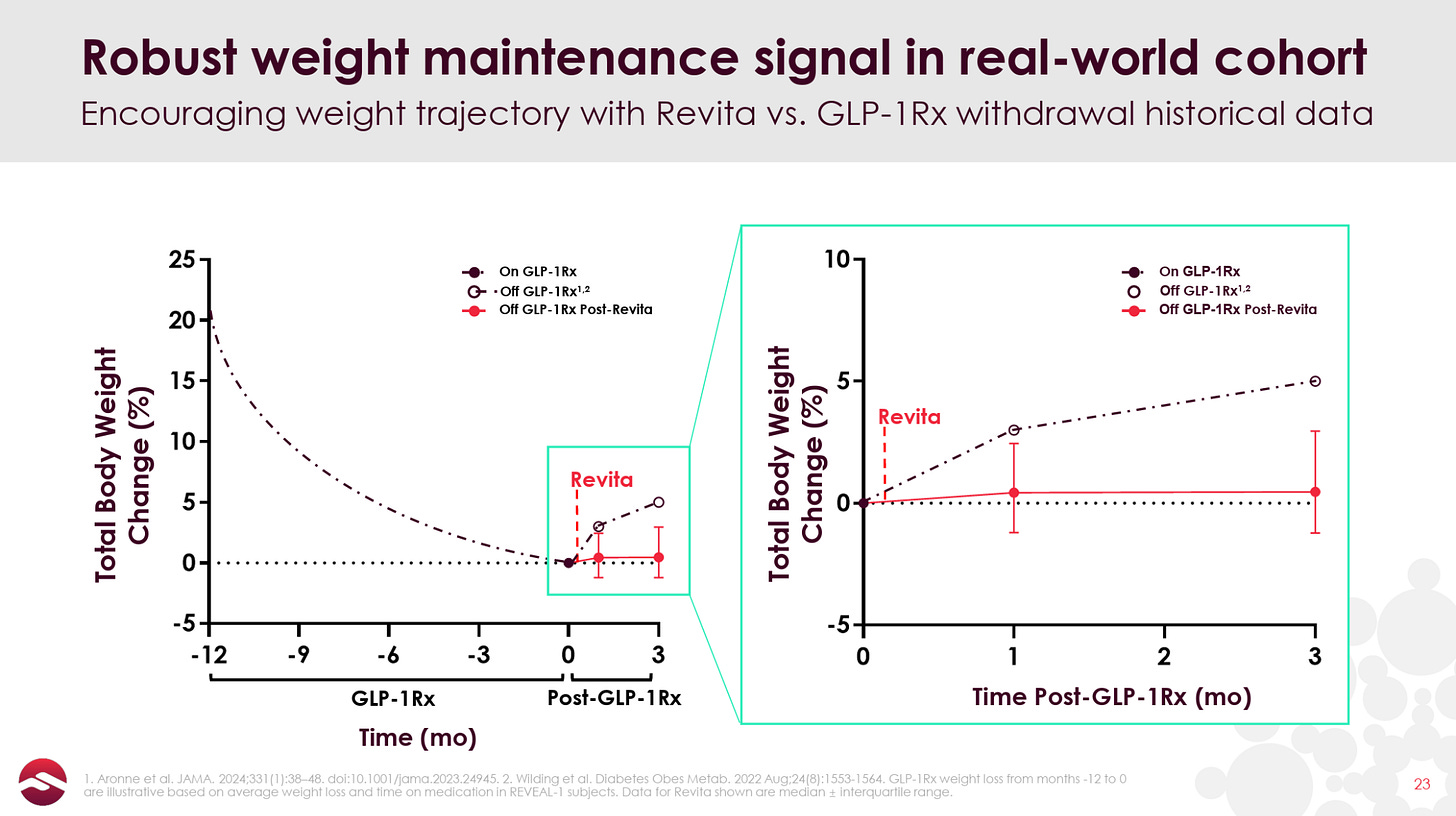
But with the advent of GLP-1s and the problem of post-GLP-1 weight rebound becoming widely recognized, Revita, if it can truly reset patients’ metabolism post-GLP-1, may have found its “killer application”. In June, Fractyl reported open-label data showing that 12 out of 13 patients maintained or saw deepened weight loss at 3 months post GLP-1 discontinuation (as opposed to 5-6% bodyweight rebound that has been demonstrated in Eli Lilly and Novo Nordisk studies).
With the obvious caveats for a small sample size and open-label data in mind (the non-Revita group in the graph above is plotted from Eli Lilly’s SURMOUNT-4 trial), these early results begin to support the metabolic reset potential of Revita. The FDA also recognized this potential, awarding Revita Breakthrough Device Designation for GLP-1 discontinuation in July 2024.
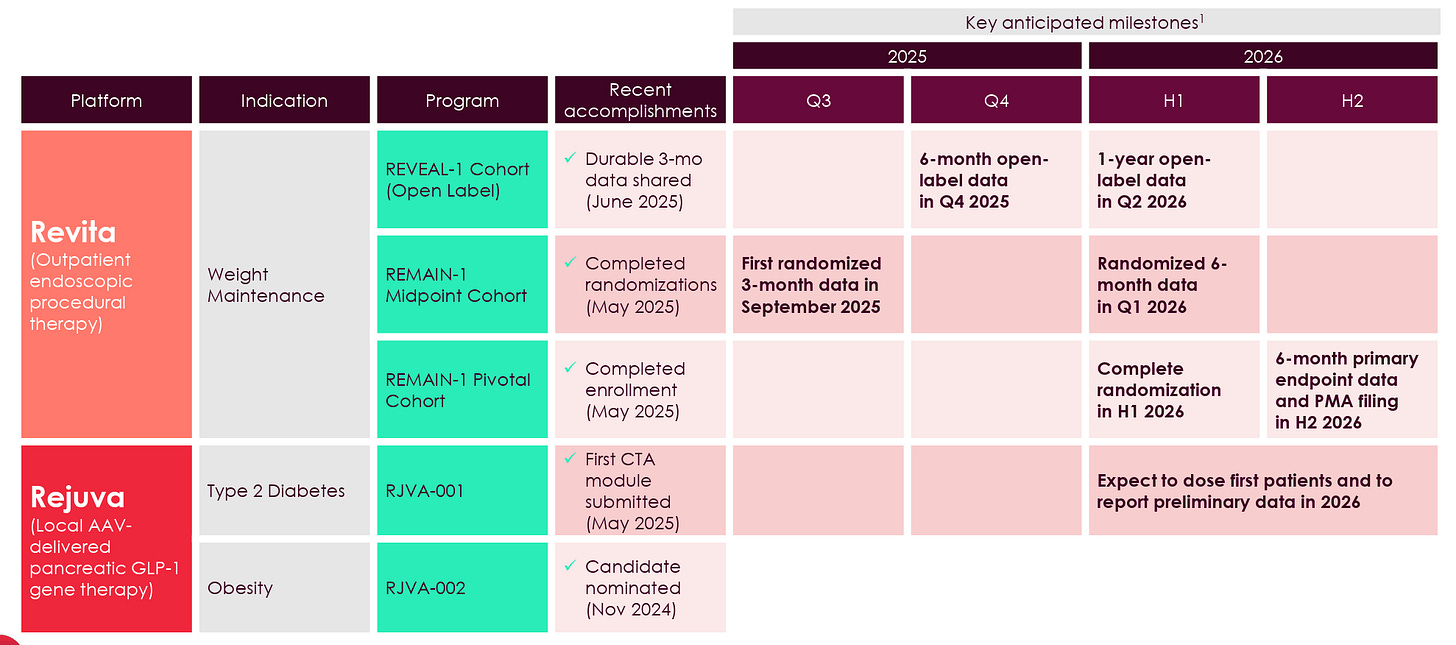
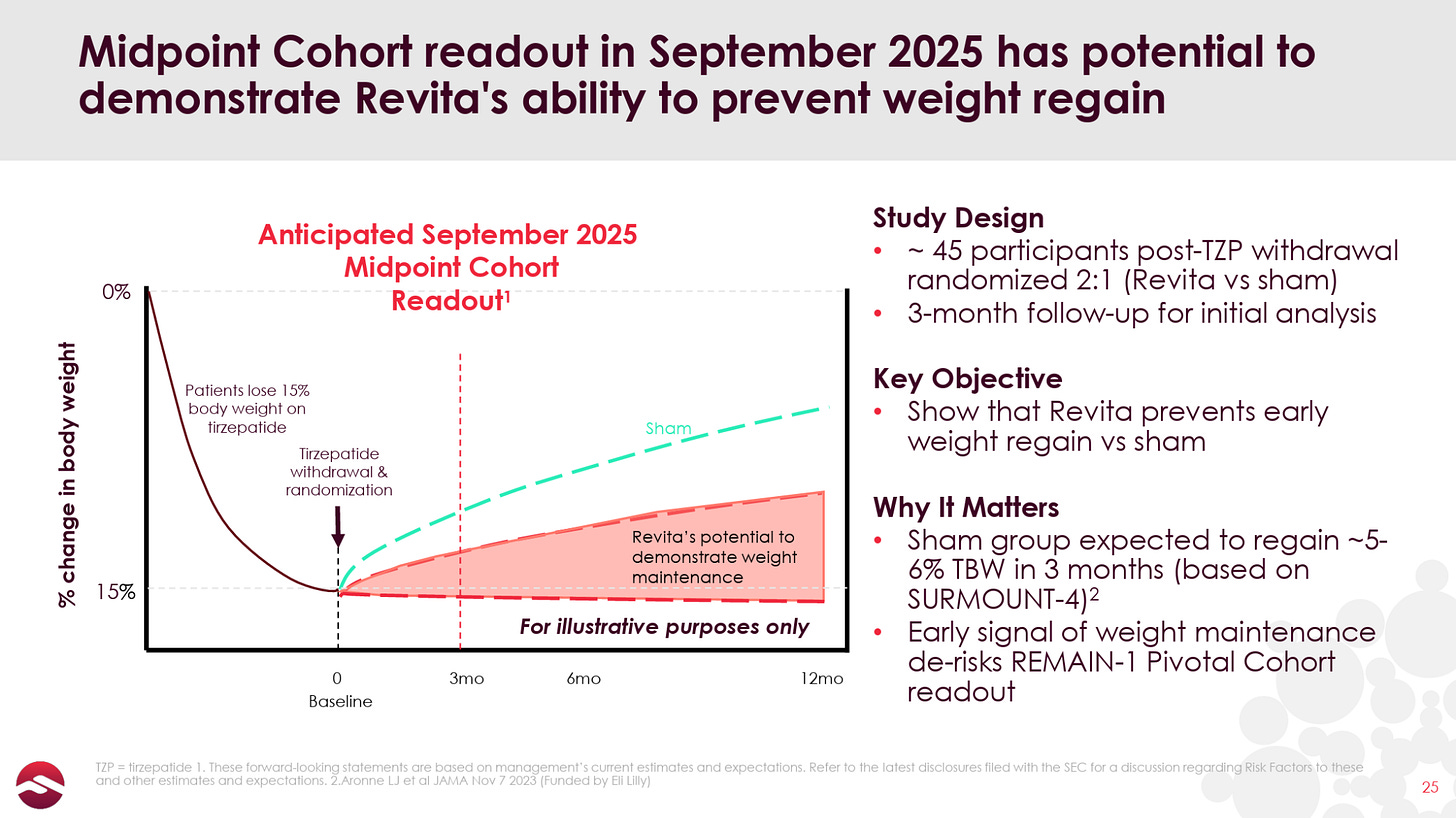
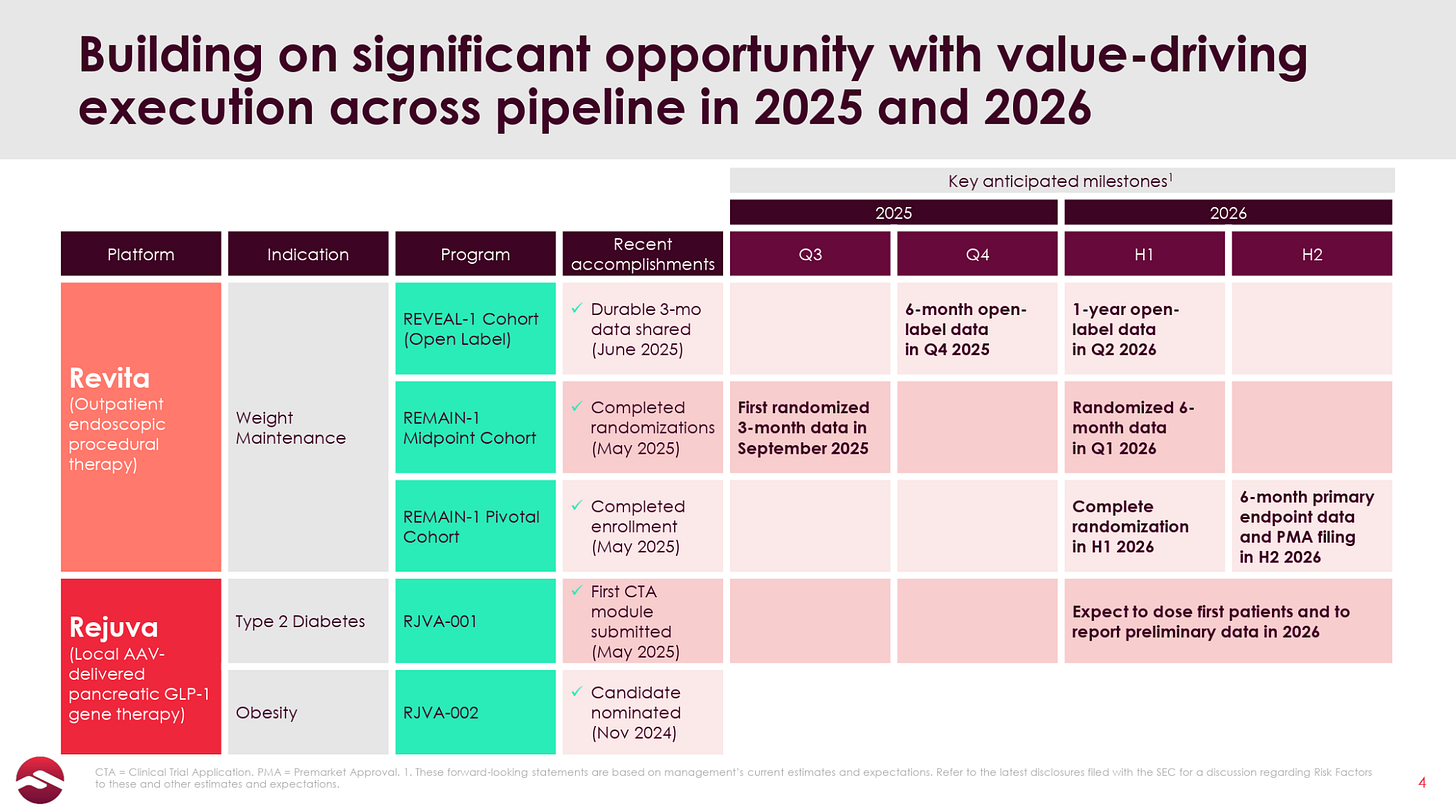
Fractyl is now conducting a 350+ patient trial in GLP-1 discontinuation called REMAIN-1, which, notably, was able to fully enroll all patients between 3Q24 and 2Q25, in stark contrast to the company’s previous REVITALIZE T2D trial which struggled to enroll a similar number of patients in over three years. The first 45-patient double-blind placebo-controlled cohort from this trial is set to report data in September.
If the imminently expected data are positive—which would look like Revita patients’ weight staying relatively stable (less than 1% body weight increase would be ideal) while placebo patients regain 5-6% of their body weight—I (and perhaps the market) would start to see Revita as a unique, one-time, orthogonal addition to the massive GLP-1-driven T2D and obesity space.
Additionally, if the data are positive, the company will be set up to build momentum throughout a slew of additional readouts from its multiple ongoing patient cohorts expected over the next 12 months, culminating with the pivotal 6-month data from its 315-patient cohort expected in the early part of the 2H26.
Fractyl recently raised $23 million, which, due to its high ~$20 million per quarter burn rate, only gives it cash runway through the 1Q26, though this will include the 6-month data readout from the same 45-patient cohort (and longer-duration open-label data). While this is a relatively short runway, the deal also included an additional $23 million tranche of warrants that are callable following the September data release, which should extend the company’s runway to(wards) the pivotal readout in early 2H26, after which the company plans to file a PMA with the FDA also in 2H26. We note that the company will likely have to raise additional capital around mid-2026, which could be an overhang on the stock if the data are not sufficiently exciting.
At a post-financing market cap of less than $70 million, and with extremely low trading volume over the past few weeks despite the upcoming readout, we think the market is overlooking Fractyl. While Revita’s lacklusterness as a standalone treatment in T2D/obesity initially gave us some pause, the encouraging early open-label data (on top of data in diabetes patients) suggests Revita has real promise as the only product in development for solidifying GLP-1 improvements. While the data due in the coming weeks is probably unlikely to cause a dramatic move in the stock price, it could get Fractyl on the radar screen of more investors and drive a gradual re-rating to a significantly higher valuation.
Weight Regain on GLP-1s
Many that follow medicine/biotech are aware of the rebound effect that occurs after GLP-1 discontinuation. In some sense, GLP-1s are such amazing drugs that losing weight is no longer the challenge, but rather being able to keep that weight off.
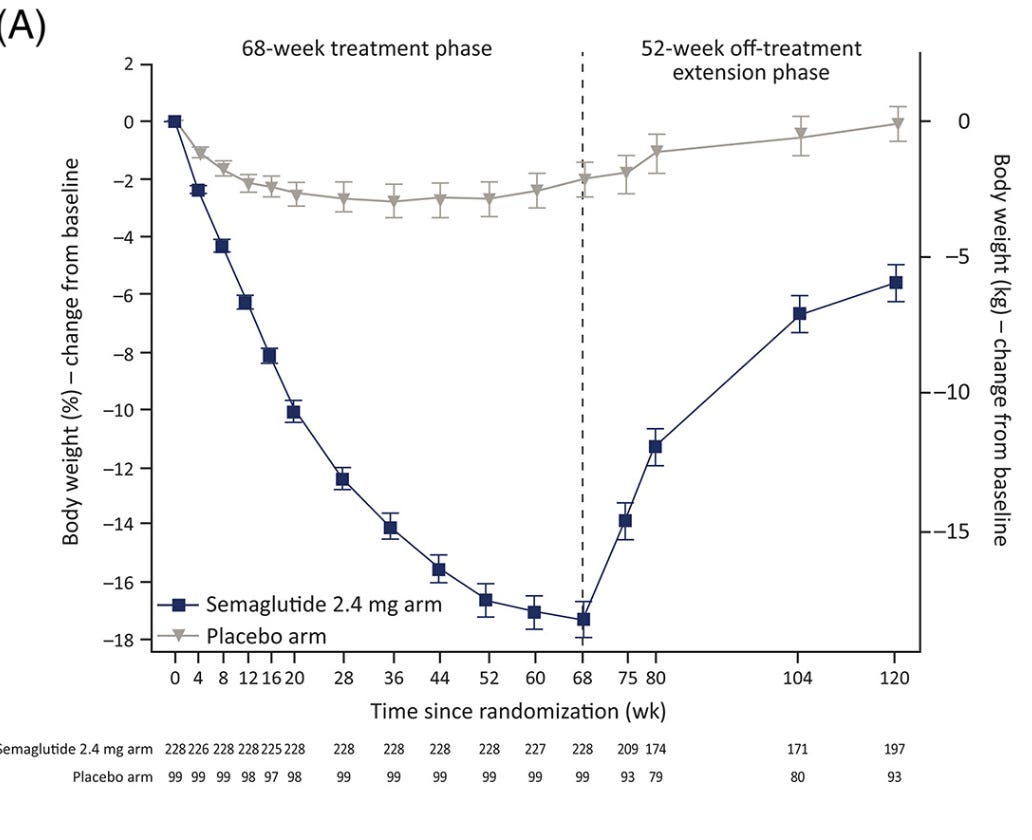
There have been a few large studies measuring the GLP-1 discontinuation rebound effect. The SURMOUNT-4 trial treated 670 patients with tirzepatide for 36 weeks, during which they lost an average of 21% of their bodyweight, then took half of the patients off of tirzepatide and observed them for 52 weeks. Over that time, the discontinued patients gained back 55% of the weight that they had lost (equivalent to a 14% bodyweight gain), while patients remaining on tirzepatide continued to lose weight.
Notably, about half of the weight regain occurred within the first 16 weeks post-discontinuation, similar to the 12-week period that Fractyl will be reporting data for this month.
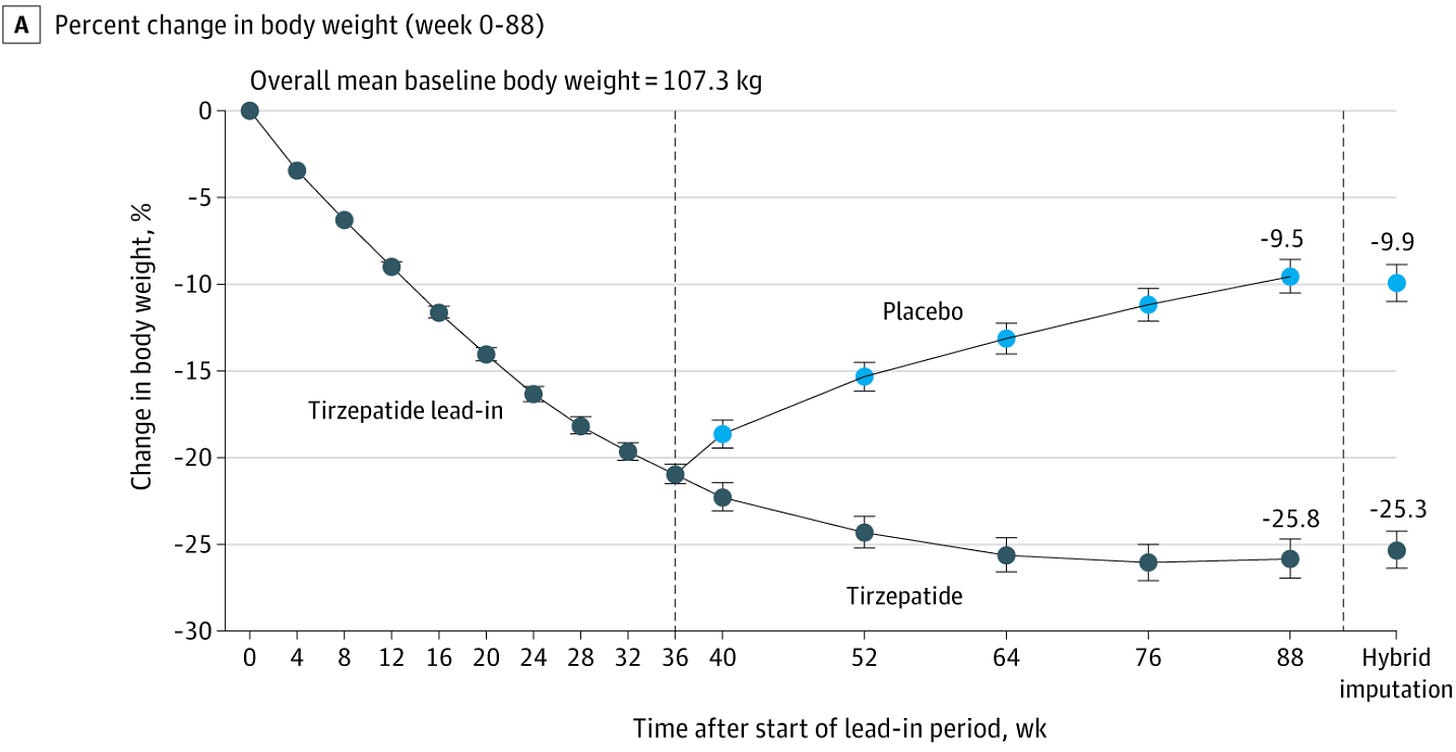
The results were similar in Novo Nordisk’s STEP-1 extension trial, which showed that patients who initially lost an average of 17% of their total body weight over 68 weeks on semaglutide regained 64% of the lost weight over the next 52 weeks after discontinuing the drug.
Again, you can see about half of all the rebound weight is regained in the first 12 weeks post-discontinuation. This rapid weight regain shortly after GLP-1 discontinuation is consistent with Fractyl management’s commentary and anecdotes from many patients saying that they feel incredibly, ravenously hungry and experience constant “food noise” after discontinuation.
This weight rebound effect has a number of consequences:
Patients lose weight progress, get demoralized, return to a less healthy state, etc.
Insurers wasted money only to have patient regain weight
If patients want to maintain the weight loss by staying on GLP-1s indefinitely, insurance companies effectively pay an annuity to GLP-1 companies, with no plan for getting off.
Some patients are afraid to go on a GLP-1 in the first place for fear they won’t be able to come off.
The fact that the most aggressive weight rebound is seen in the 12-16 weeks post GLP-1 discontinuation makes Revita’s September readout a key incremental data point in our minds.
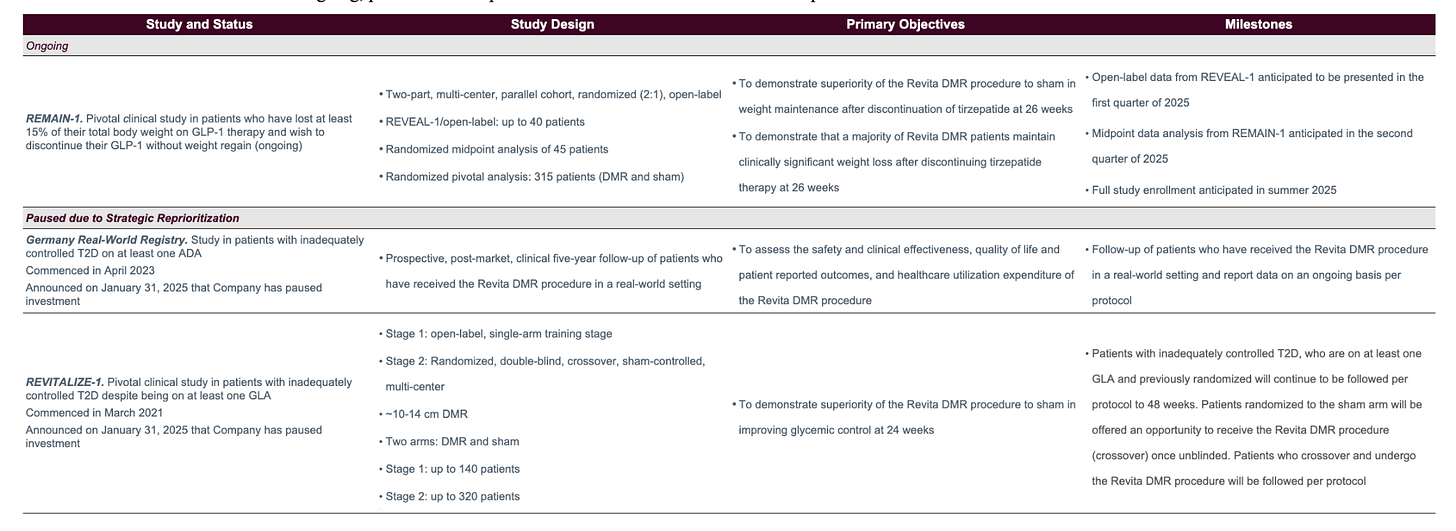
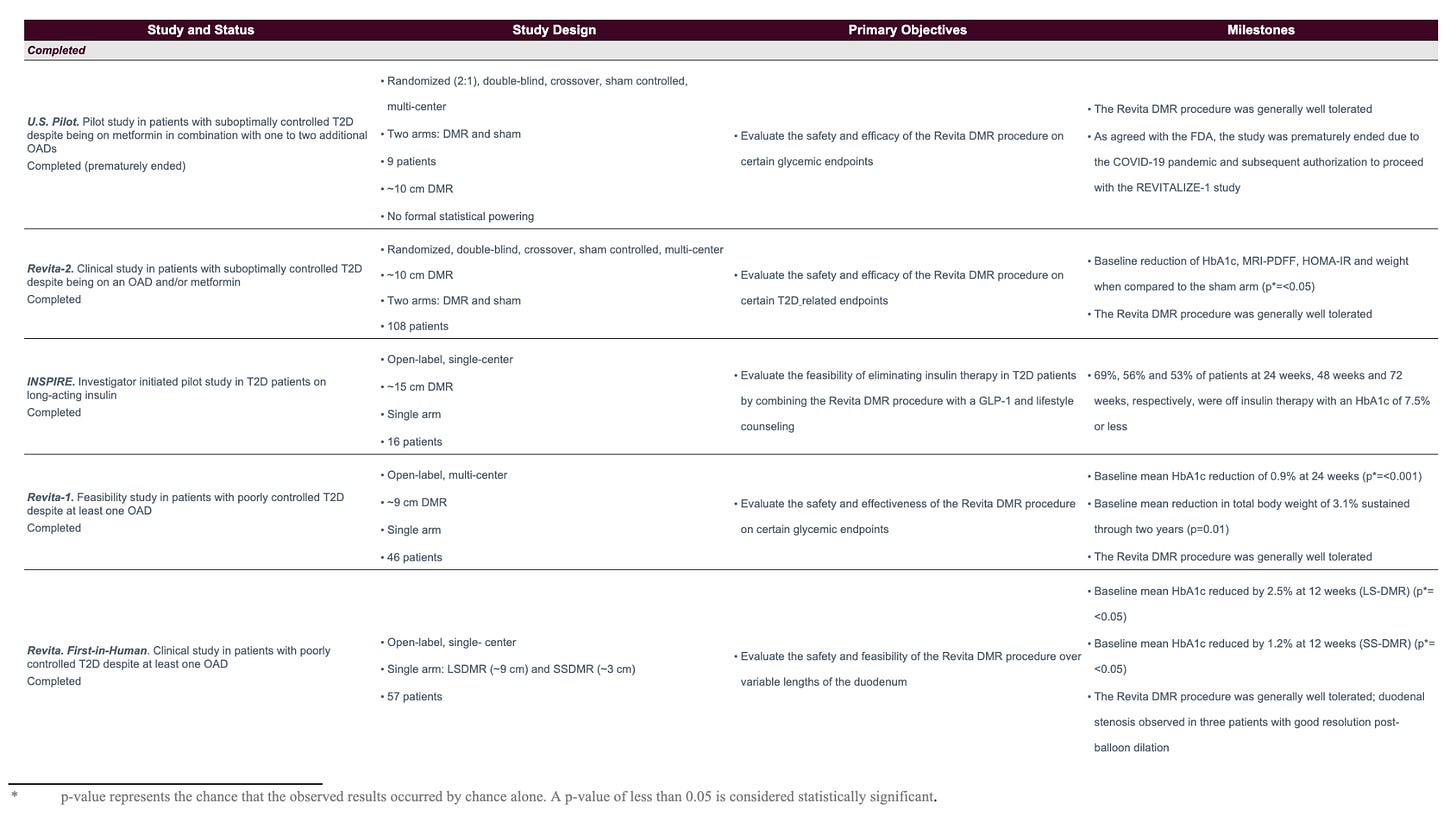
Historical Revita Data
Beginning in 2013 with the company’s first-in-human trial, Revita has been trialed and published data in over 250 patients to date, the large majority of which were T2D patients. Below is a summary of all of the trials that Fractyl is either currently conducting or has conducted in the past in reverse chronological order:
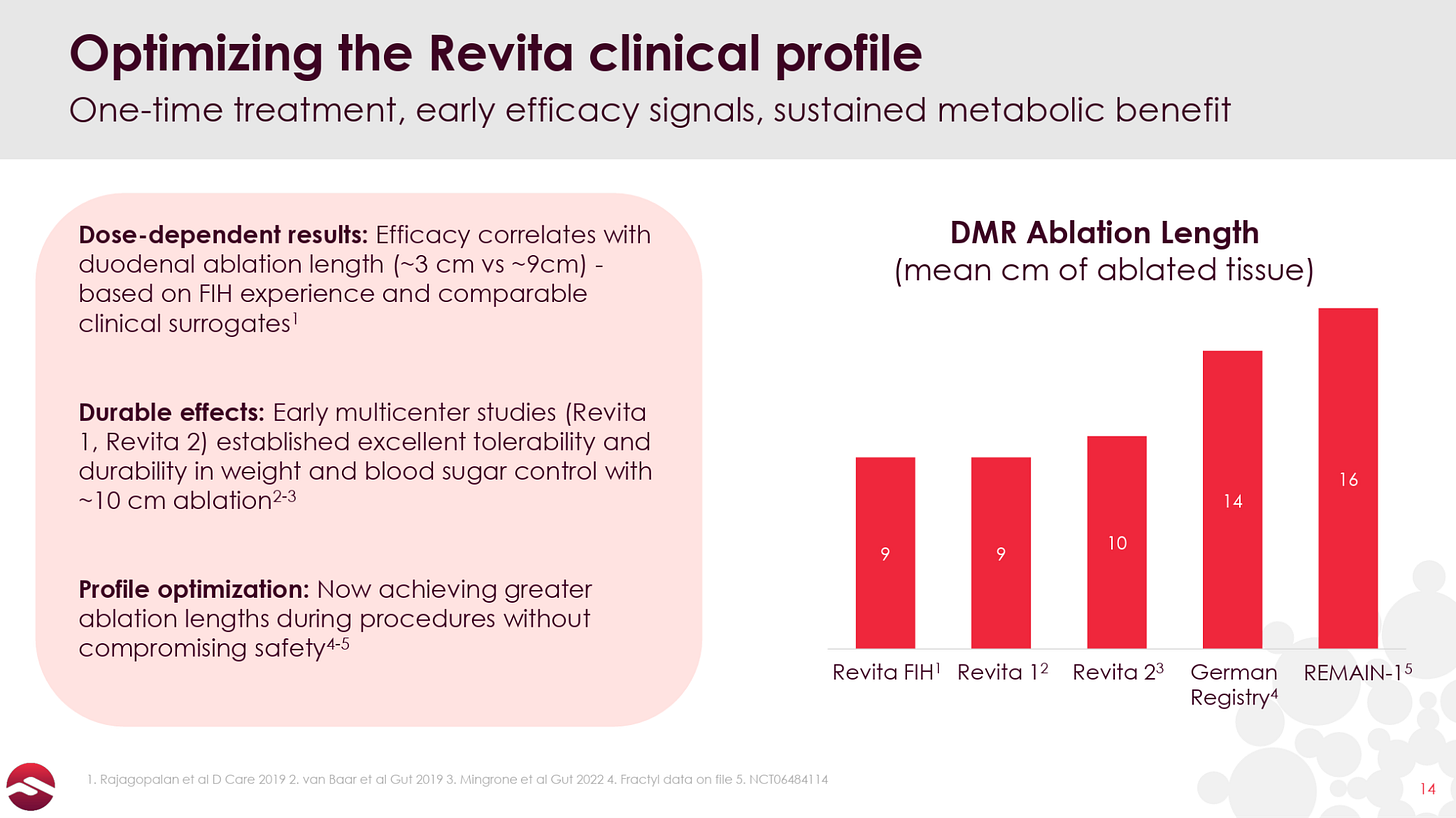
If you look into the results of each individual trial (which you can do in prior 10-Ks), you can see that the results range, with weight loss ranging from 3% to 10% (with essentially 100% of weight loss occurring within 3 months of the procedure) and HbA1c reduction aggregating around 1-1.5 percentage points.
The data essentially ranges from solid to mediocre, and is affected by different patient cohorts across the trials and, the company states, the fact that greater effects are seen with larger ablation areas. The company was only ablating up to nine cm of duodenal tissue in its first couple trials, while the REMAIN-1 trial ablates 16 cm.
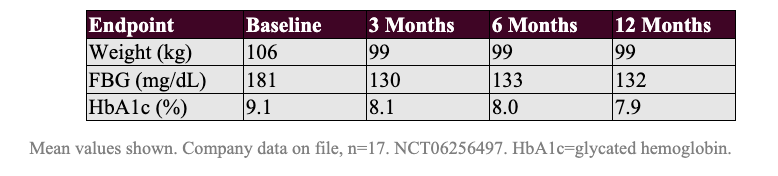
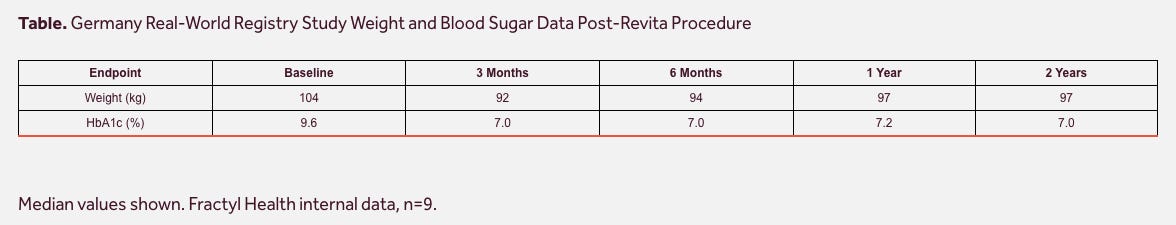
Given that the German Real-World Registry study uses a similarly-long ablation length to REMAIN-1, it is interesting to consider the data from the 17 patients that had reached one year of follow up as of early 2025:
These patients lost 6.6% of their bodyweight by Month 3 and sustained the weight loss to Month 12, and reported a 1.0% HbA1c reduction at Month 3 that deepened further over time.
Zooming in further on the German Registry data, in August 2025, the company released data from the first 9 patients that had reached 2 years post-procedure.
These patients lost 9.6% of their bodyweight and saw an impressive 2.6% HbA1c reduction 2 years after being treated with Revita. You will notice that patients gained weight from 3 Months to 2 Years, which isn’t ideal, though we note that weight remained stable from 1 Year to 2 Years post-procedure.
While efficacy varies across the different studies, Revita clearly has at least some activity in metabolic diseases. Perhaps the most interesting aspect of this data as it pertains to Revita’s prospects in GLP-1 discontinuation, though, is that the weight loss and HbA1c reductions are seen rapidly (the first observation period, usually three months) and critically, in the large majority of the data, appear to be durable out to 1 or even 2 years. So while the absolute weight loss delivered in these trials in T2D patients is not overly impressive, the data are congruent with the thesis that Revita could drive a durable metabolic reset.
Open-Label GLP-1 Discontinuation Data
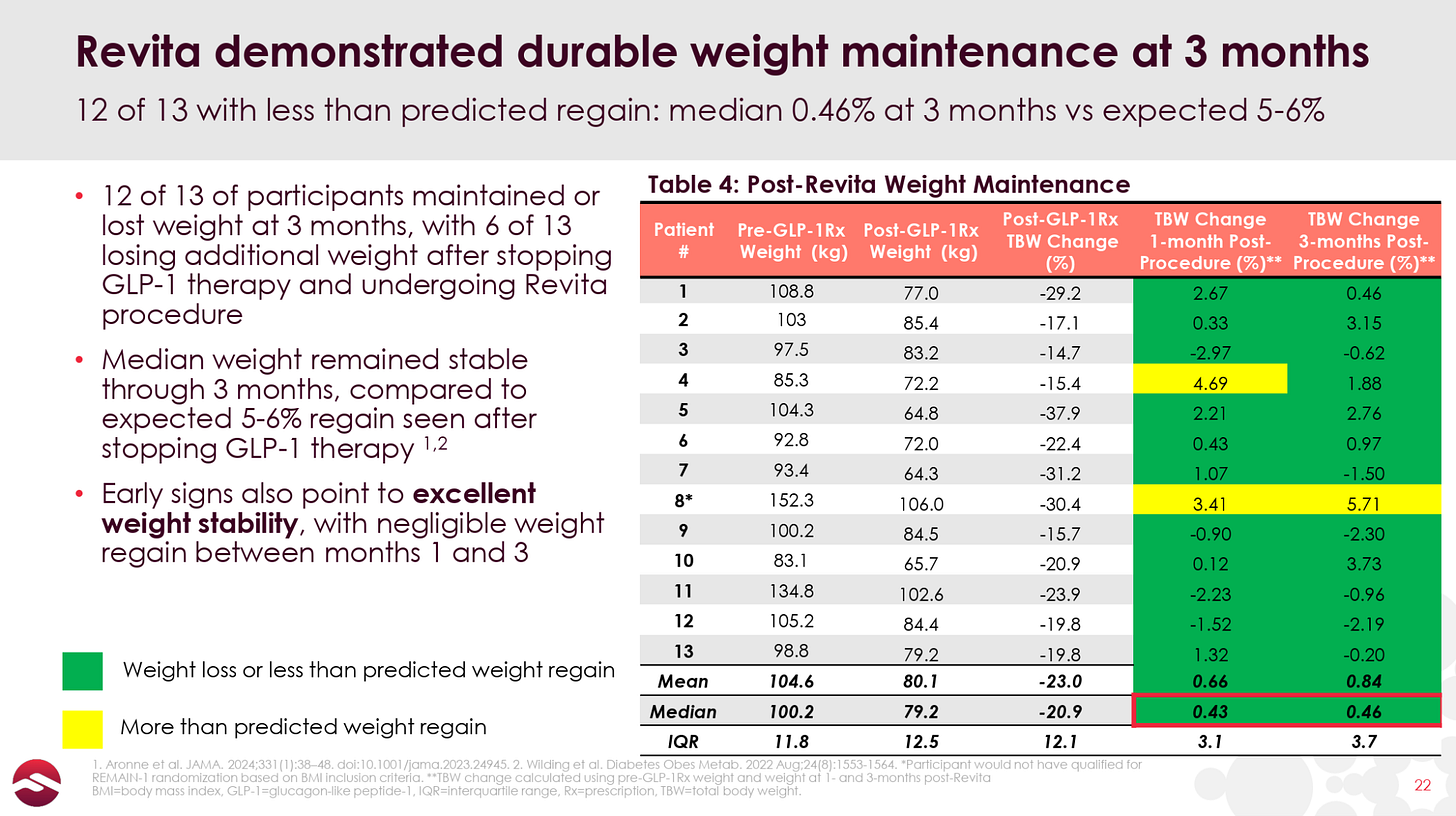
In June, Fractyl gave the first glimpse of data for Revita in GLP-1 discontinuation, announcing 3-month results from 13 GLP-1 discontinuation patients that showed a median weight regain of 0.46%. Here are the detailed data:
As you can see, there is one patient that had the expected 5-6% of bodyweight regain and another two patients that gained back 3%+ of their body weight at Month 3. Other than that, most patients performed quite well, with an impressive 6 of the 13 patients losing additional weight post-GLP-1 discontinuation and 9 of 13 patients regaining less than 2% of their bodyweight or better. We also note that 6 of the 13 patients had an improving effect from Month 1 to Month 3, indicating that the benefits of Revita may take a couple of months to fully manifest.
Here is the data plotted against the 5-6% total bodyweight gain from Lilly’s SURMOUNT-4 trial:
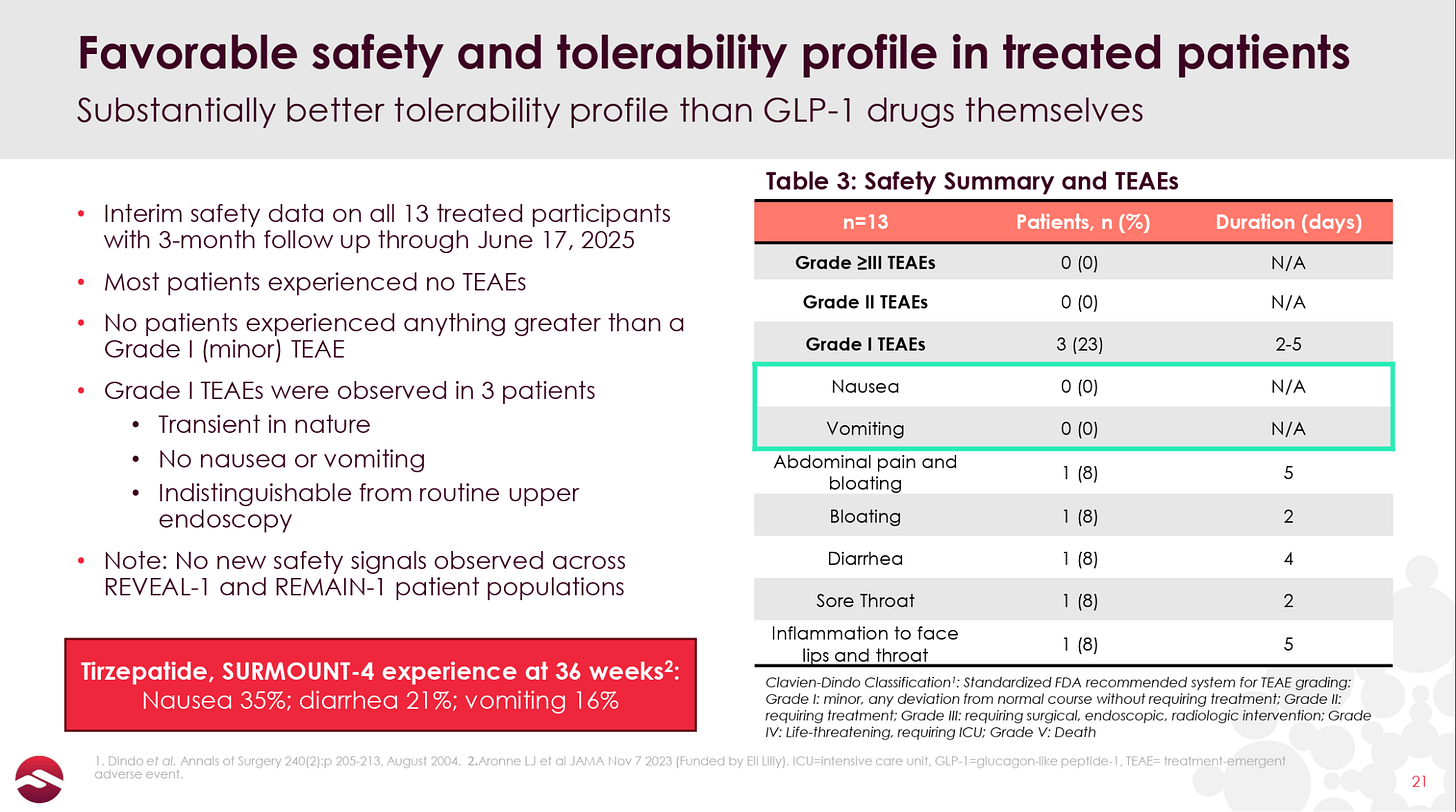
It is also worth noting that Revita’s stellar tolerability profile was maintained in the open-label results:
The ability to offer patients a one-time, pain-free, nausea-free solution to getting off of their GLP-1 would likely be a key selling point for potential patients.
While these small, open-label results are extremely preliminary, they give some early indication that Revita may have the ability to effectively “lock-in” most of the weight loss gains of GLP-1s, a much more impressive and socioeconomically valuable effect than its results in T2D.
September Data: The Bar for Success
While the readout expected this month is only 3-month data in 45 patients, Revita’s rapid onset of action and the rapidity of post-GLP-1 weight rebound should provide plenty of opportunity for Revita to show significant separation. Management has commented that its goal is to deliver 50% better results than what would be expected from tirzepatide withdrawal normally (i.e. 2-3% bodyweight regain vs. 5-6% expected).
Obviously this is a conservative goal versus Revita’s open-label data, but it gives some leeway for natural variance, and signals that management believes the study is a success even if the Revita arm of the trial doesn’t fully mirror the open-label result. While we believe a 50% improvement versus placebo would still be a technical and clinical success, we are not sure the investment community would be thrilled.
Given the limited open-label data, we would conservatively hope for bodyweight regain of around 1% or better at 12 weeks (vs. the ~5% expected for placebo), which we believe would be considered a success by both the scientific and investment communities. If the data were to be in the 2-3% bodyweight regain at 12 weeks, Revita would likely still be a viable medical device candidate, though less exciting, and possibly of significantly less interest to investors.
Overall, it is difficult to predict what the market considers to be the line between success and failure in this trial (assuming there are people watching this trial closely at all). In order to really get on the radar screen of investors that are not currently aware of the company, we believe the results likely need to be in the 1% bodyweight regain or better range.
Next 12 Months
Following this month’s results, Fractyl has a series of other readouts from the three different cohorts expected in the next 12 months, including:
6-month data from the open-label patients in the 4Q25
6-month data from the 45 placebo-controlled patients in the 1Q26
1-year data from the open-label trial in 2Q26
6-month data from the 315+ pivotal cohort
Over the next 12 months, Revita will be pretty comprehensively characterized as a treatment for GLP-1 discontinuation patients, culminating in a relatively near-term opportunity to submit a PMA in the 2H26. The upcoming 12-week results serve as a proof-of-concept, setting both the expectations and the level of investor interest/excitement for these upcoming readouts.
Market and Commercialization
To put some numbers to the market size and opportunity for Revita in GLP-1 discontinuation patients:
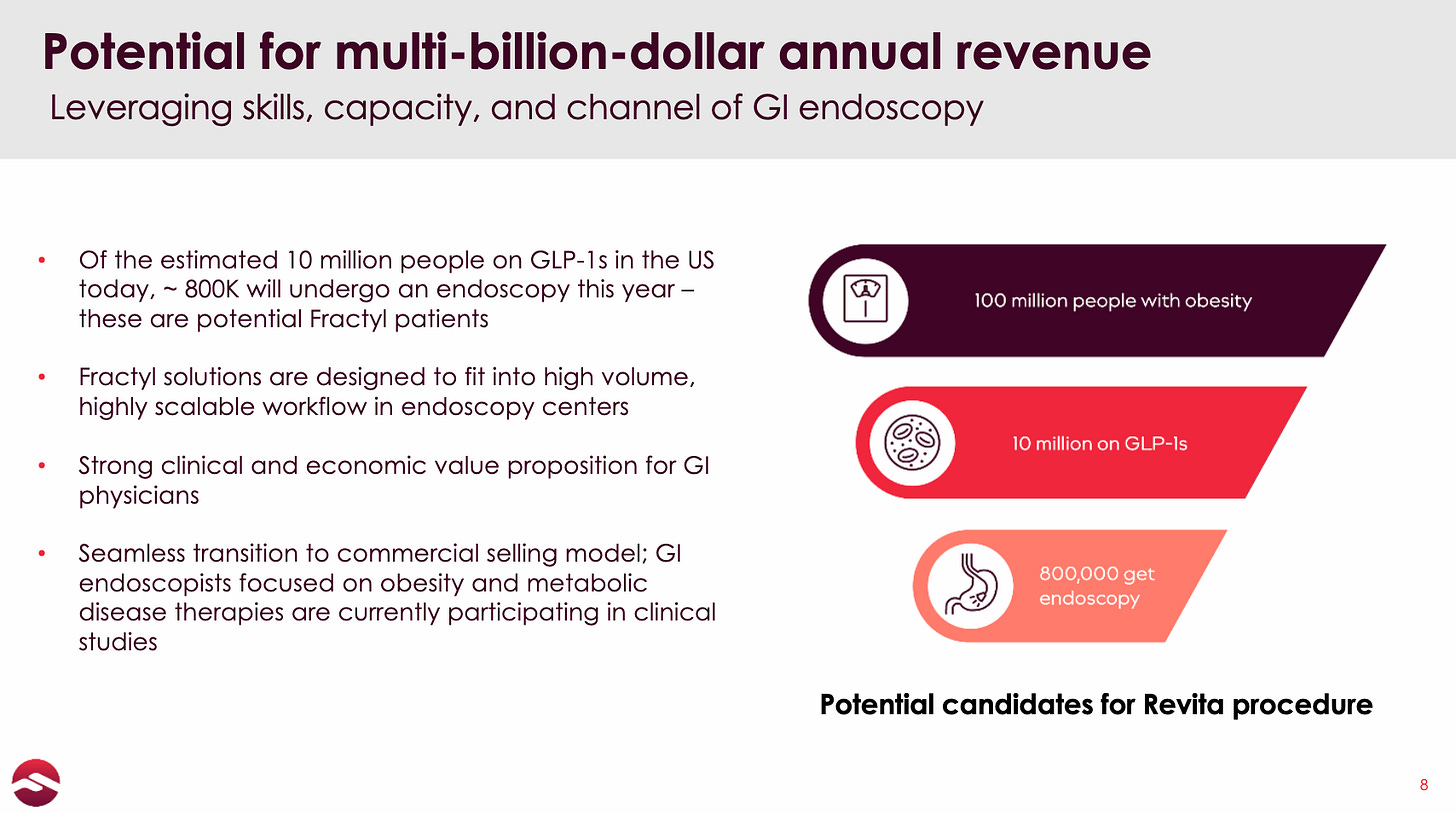
There are 100 million people with obesity in the US
There are ~10 million patients on GLP-1s
More than 50% of patients will discontinue GLP-1 therapy within 3 months
85% of GLP-1 discontinuers will regain weight
More than 250k bariatric surgeries (significantly more invasive than Revita) are performed each year
There are more than 20 million endoscopies performed each year in the US, including over 600k advanced endoscopic procedures
Of the ~10 million patients on GLP-1s, ~800k will get an endoscopy for various reasons
Revita is a ~45-minute out-patient procedure (with little/no side effects) that Fractyl states can be easily performed by advanced endoscopists at an estimated 2,000 to 4,000 sites in the US today. It takes just a couple of patient walkthroughs for physicians to become proficient in the procedure, and there are already 32 clinical sites performing Revita procedures as part of the REMAIN-1 trial, on top of the 51 US sites that were performing Revita procedures as part of the paused REVITALIZE trial in T2D (though many of these sites overlap). The company also signed a letter of intent with Bariendo in June to potentially partner on providing Revita through its network of endoscopic obesity treatment centers.
Fractyl also has the interesting option of potentially positioning Revita as an add-on procedure to the 800k GLP-1 patients that will undergo an endoscopy each year. The kicker here is that, according to the company, most gastroenterologists/anesthesiologists already require that patients stop GLP-1 medicine prior to an endoscopy due to the slowed gastric emptying. This could be a natural “GLP-1 off-ramp” offered to patients that allows Revita to seamlessly penetrate its market opportunity.
On the payor side, in an interview with Inside Precision Medicine, CEO Harith Rajagopalan stated that he thinks payors are desperate to be able to deprescribe GLP-1s, which makes intuitive sense since many patients will either discontinue (rendering the money essentially wasted) or continue to use GLP-1s indefinitely (a GLP-1 annuity) with no plan of how to get off and cement the health gains. Fractyl has indicated that early internal discussions surrounding Revita’s pricing are settling around the equivalent of 18 months of GLP-1 therapy ($500-$1,000 per month), which, if the durability of Revita can be proven to insurers, should be a welcome long-term solution.
Recent Financing
Fractyl recently completed a $23 million equity offering that also includes two additional $23 million warrant tranches (with exercise prices of $1.05 per share), one of which is callable following the data expected this month if Fractyl’s trading price exceeds $1.37/share.
Fractyl guided that the $23 million will allow it to get through the release of 6-month data from the 45-patient REMAIN-1 cohort in the 1Q26, and we would expect the runway to extend to the full 315-patient 6 months results in early 2H26 if the first tranche of $23 million warrants is able to be called and exercised (though that would require the stock gaining some momentum driven by the upcoming results).
The named investors in the financing included healthcare-focused investors Nantahala, ADAR1, and SilverArc, as well as Second Line Capital and 683 Capital. ADAR1 was a 5% owner of Abivax (ABVX) going into the August data readout that saw the stock soar 600%+, not that that means anything about its investment in Fractyl.
Fractyl’s cash situation is definitely tight, and puts extra pressure on the demonstration of Revita’s value over the next couple quarters. If data is less than stellar, the capital overhang could be a serious problem for the stock.
Risks/Questions to Ask
Revita’s Long, Winding Road
Revita began its first trial in T2D in 2013 and received its CE mark in Europe in 2016, but never commercialized in Europe to any meaningful extent because of the challenging regulatory and reimbursement statuses of the individual countries. It did gain reimbursement status in Germany in 2022, after which it began a pilot commercialization effort and its German Real-World Registry trial, both of which gained some minimal traction before being paused as part of the reprioritization towards GLP-1 discontinuation in January 2025. The company also decided to pause the enrollment of its primarily US-based REVITALIZE trial in T2D after enrolling for over three years. While the shift to GLP-1 discontinuation is clearly the right strategic decision, Revita’s long history and lack of real success in T2D makes it seem like an unlikely blockbuster medical device candidate at first blush.
Unproven Efficacy
There is very limited data available for Revita in GLP-1 discontinuation patients at present. Beyond the small 13-patient open-label data, we are using Revita’s results in T2D patients to infer about GLP-1 discontinuation patients. The durability of Revita’s weight maintenance in post-GLP-1 patients, which will be crucial to its viability as a treatment, also won’t be known for at least a couple more quarters.
Open-Label Data
While the open-label data looks incrementally positive, and the company includes a detailed breakdown of the data in its slide deck, we find it a bit odd that Fractyl released the 3-month open-label data from only the first 13 patients in the open-label study, rather than waiting for the full 22 patients enrolled in the study to reach 3 months.
Data Readout Pushbacks
Though it could probably be blamed partially on financial constraints, Fractyl does have a history of pushing back the expected readouts of its trials. For example, it originally announced that it would report the open-label REMAIN-1 results in the 4Q24 (it came in June of 2025), and the REVITALIZE trial (now paused) was also supposed to report results in 2024. That said, with all cohorts of REMAIN-1 already fully enrolled as of May 2025, the guided timeline over the next few quarters should be accurate.
Oral GLP-1s
While we don’t necessarily think the oral GLP-1 drugs currently in development represent a substantial threat to Revita, primarily because of their side effect profiles and that patients/physicians/insurers will still want a long-term solution, there could be more convenient, less nauseating oral drugs developed that at least some portion of patients would likely opt for over a procedure like Revita.
Digital Health Results from Omada Health
We are aware of a small, partially flawed though still interesting study conducted by Omada Health that found that its digital health program was able to essentially eliminate weight regain in patients discontinuing their GLP-1 medication. If these results were to be replicated, it could represent an alternative to an invasive procedure like Revita, though we note that a large number of patients will not be either physiologically or psychologically capable of preventing weight regain through behavior alone.
Fear of Endoscopic Procedure
While it is essentially equivalent to a routine endoscopy, some patients will never consider Revita simply because it involves anesthesia.
Updated GLP-1 Practices
While there will still always be a number of patients that abruptly stop GLP-1s for financial, clinical, or other reasons, the idea of very slowly titrating down a GLP-1 instead of just discontinuing treatment will likely continue to become more and more prevalent, potentially improving GLP-1 discontinuation outcomes.
Dilution Risk
As we discussed in the financing section, there is potential dilution from the $23 million of warrants that could be exercised if Fractyl’s stock is trading above $1.37 after this month's data, which would only get the company to the 2H26. A worse outcome would be that the stock price never gets to $1.37 and the company needs to do a raise at sub-$1 prices.
Conclusion
Fractyl sits at a critical juncture: It has a lot of interesting yet incomplete data to suggest that its Revita procedure could be a viable metabolic reset treatment to cement GLP-1 weight loss, and is expected to report the first of a series of important data readouts that will characterize Revita’s ability to address this large opportunity imminently. While the company’s sub-$70 million market cap as it approaches its first placebo-controlled readout in this large and novel indication has caused me to question if I am missing something, I have looked under pretty much every stone I could find (with the results conveyed in this piece), and the recent investment from respectable healthcare investors lends some incremental solace. While the financing situation will continue to be an ongoing concern and will put extra pressure on the company to report exciting data, Fractyl is underfollowed and potentially just ~12 months away from being able to file a PMA in the massive and as of yet completely untapped GLP-1 discontinuation market.



it just need to clear the duodenum and there's already a lot of endoscopy done, why then people pay for GUTS device while they could just do endoscopy even today?