Key Points
Current $1.2 billion valuation is unjustifiably low; conservative fundamental valuation yields $4+ billion fair value.
AXS-05 has strong clinical data and a differentiated mechanism of action.
Addressing underserved 20 million-patient depression market.
Introduction
Axsome Therapeutics (NASDAQ:AXSM) currently represents a special situation. A series of unexpected regulatory delays have resulted in an unjustifiably low $1.2 billion market capitalization for the stock. A conservative estimate of sales and the assignment of a well-below-average sales multiple yields a $4+ billion fair value, as is discussed in detail in the Valuation section below. Our two-year price target is $159/share (+400%).
Of course, that valuation is based on the assumption of clinical success, FDA approval, and successful commercialization. And while Axsome has a deep and intriguing portfolio of mid-to-late-stage clinical programs that mitigates its downside risk, its lead program, AXS-05 in major depressive disorder (MDD), is by far the largest market opportunity for the company and the most important growth catalyst for the stock.
The safety and efficacy (i.e. clinical success) of AXS-05 has been clearly demonstrated in multiple Phase 2 and Phase 3 studies which have been discussed at length in past Seeking Alpha articles. AXS-05 exerts rapid-onset antidepressant effects (absent in standard SSRI antidepressants) with durability beyond 12 months of treatment. The data is compelling, and can be viewed in an easy-to-understand poster format on the publications page of Axsome’s website.
Despite clinical success, FDA approval has eluded Axsome. A series of delays and slow, opaque correspondence with the FDA has pushed AXS-05’s approval decision more than eight months beyond its original late-August 2021 PDUFA date.
It has been a long, frustrating whirlwind, and it seems many investors have been scared or frustrated into sitting on the sidelines, or even into shorting the stock (15% short interest). Not only have the constant delays damaged the market’s opinion of Axsome’s integrity and internal organization, but they’ve also made it difficult to ascertain and focus on the true value of the stock. The end result is a special situation, in which Axsome trades at a $1.2 billion market cap that is fundamentally baseless. Those who can cut through the zig-zagging updates and headlines to focus on Axsome’s underlying technology and portfolio will find a compelling value proposition.
In this article, we will disregard all of Axsome’s programs that are not AXS-05 for the treatment of MDD (Sunosi, AXS-07, AXS-05 for Alzheimer’s agitation, etc.), most of which are promising. The market’s mispricing of Axsome is so extreme that a convincing valuation-based argument for $159/share can be made based on the AXS-05 in MDD program alone.
Recent History of AXS-05
On 4/26/21, Axsome announced that AXS-05’s NDA application for MDD, which holds a Breakthrough Therapy Designation (BTD; awarded in March 2019), had been accepted by the FDA and granted Priority Review status. It was assigned a PDUFA date for 8/22/21, which, strangely, was only four months away from the FDA’s acceptance of the NDA (Priority Review represents the intention of the FDA to complete the review process in six months versus the usual ten).
However, in the 2Q21 earnings released on 8/9/21, Axsome announced that the FDA had identified two deficiencies in the chemistry, manufacturing, and controls (CMC) section of the AXS-05’s application that would inactivate the PDUFA date. While Axsome was originally not given the specific details of the deficiencies, they were eventually revealed on the 3Q21 earnings call to be related to “analytical methods”. CEO Herriot Tabuteau explained that the deficiencies related to the quality assurance procedures of AXS-05’s manufacturing process, and that he believed the deficiencies were easily addressable.
On 1/18/22, Axsome announced that it had “provided a response” to the FDA, addressing the deficiencies, and had not been made aware of any other issues with the NDA. Then, a month and a half later, in its FY2021 earnings release (3/1/21), Axsome announced that the agency had acknowledged receipt of that response. And the tedious process continued.
On the 3/26 Sunosi acquisition call, Herriot announced that recent communications with the FDA made it clear that the deficiencies were resolved. Since then, the standing guidance has been that Axsome will provide an update via press release upon entering into “labeling discussions” with the FDA. This guidance was repeated whenever analysts attempted to get more information about AXS-05.
“Labeling discussions”, i.e. discussions with the FDA regarding what will go on the product’s label, which is also known as its U.S. Prescribing Information (USPI; more info here), is one of the final steps in the FDA’s review process, taking place after the drug’s safety and efficacy are confirmed. They typically occur about one month prior to the PDUFA date (per Herriot on the FY21 earnings call).
So, the stage was set: the next piece of news investors got regarding AXS-05 would either effectively affirm its approval, or introduce some new unexpected setback.
The next piece of news was neither, technically. On 4/19, Axsome filed an 8-K that stated it had “received and agreed to Postmarketing Requirements/Commitments” proposed by the FDA.
Postmarketing requirements and commitments are studies a company agrees to conduct on a drug following its commercial launch. They are conducted for various reasons, including to explore interactions with other drugs or to test specific patient subpopulations. This study found that 55% of drugs had at least one postmarketing commitment at initial approval, and that only 10% of those commitments were actually for new clinical trials.
Also included in the 4/19 8-K, Axsome announced it now anticipated an FDA action on AXS-05 in the 2Q22 (i.e. within the next two months). For a company that has taken a very conservative approach to guidance, this was significant.
So, while not technically “labeling discussions”, agreement with the FDA on postmarketing commitments would seem to strongly imply that AXS-05 will indeed be a marketed drug in the near future.
Valuation
For the purposes of this valuation, we are leaving aside the 4/25 announcement that AXS-07 (Axsome’s migraine drug) would miss its 4/30 PDUFA date due to CMC issues. In the four trading days separating the AXS-05 update and the AXS-07 delay, during which the market should theoretically be pricing in a near-100% chance of approval for AXS-05, we see that shares popped only 25% on the day of the news, and finished the week up only 20% at $39/share, or $1.46 billion in market cap. For reference, Axsome traded up to $70 in anticipation of AXS-05's original PDUFA date.
The sections below describe why $1.46 billion is a vast undervaluation of AXS-05 as a soon-to-be FDA-approved, mechanistically differentiated treatment option in the underserved 20 million-patient MDD market.
Market Opportunity
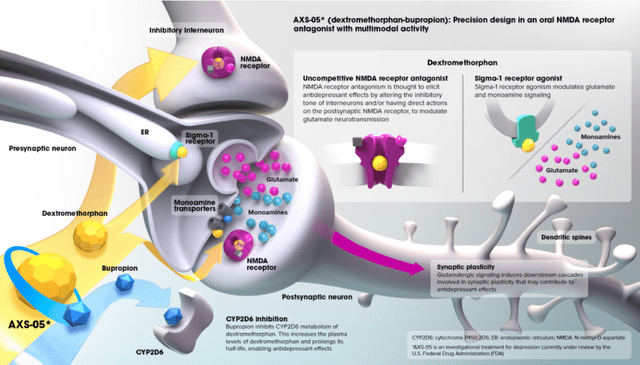
Perhaps the most important aspect of AXS-05’s market opportunity is its differentiated mechanism of action (MOA), which could provide physicians and patients with a valuable alternative to classic serotonergic antidepressants, which classically report symptom remission rates of 30-40%.
AXS-05’s active ingredient, dextromethorphan (DXM), the active ingredient in Robitussin (though DXM is quickly metabolized to dextrorphan in Robitussin), has a wide range of pharmacological effects (“multimodal”, as termed by the company). Chief among these effects is AXS-05’s NMDA (glutamate) receptor antagonism. Whether AXS-05’s NMDA receptor antagonism is truly the most prominent mechanism by which it exerts its antidepressant effects, which is difficult or impossible to know due to its pharmacologically relevant effects on numerous receptors, or whether Axsome has just decided to brand AXS-05 as an NMDA receptor for reasons discussed in the following paragraph, is unclear. Either way, AXS does indeed have significant NMDA antagonist activity.
NMDA receptor antagonism has received a lot of attention in recent years, especially in depression, as it is the mechanism by which esketamine, or Spravato (Janssen; approved in 2019), is thought to work. The approval of Spravato received massive media attention for being the first new depression treatment approved in over a decade, having a non-serotonergic mechanism of action, and showing rapid-onset antidepressant effects that persisted for multiple weeks after a single intranasal dosage.
But while esketamine is known to have powerful antidepressant effects, it was given an REMS designation from the FDA due to its abuse potential and can only be administered in a doctor’s office. There is no other NMDA receptor antagonist currently approved for depression. AXSM has the potential to make the paradigm-shifting mechanism of esketamine (or similar) significantly more accessible with twice-daily oral dosing.
While the above is an incomplete oversimplification of the pharmacology and I intend to provide a more in-depth exploration of the science in a future article, AXS-05 has demonstrated both rapid-onset and long-lasting antidepressant effects by way of a (mostly) glutamatergic mechanism of action that may appeal to a large proportion of prescribers who have not responded to traditional SSRIs.
Below are two methods we will consider to value AXS-05: a multiple valuation based on projected sales and a comparable company analysis.
Multiple on Projected Sales
Axsome quotes the MDD patient population at 19 million. Because patient population estimates vary and AXS-05 could very easily be prescribed off-label for similar, large patient population disorders like anxiety, we will use 20 million as an estimate of total addressable patient population in MDD.
Assume just 5% market penetration, AXS-05 would reach 1 million patients. It is worth noting that 13% of US adults aged 18 and older reported using an antidepressant between 2015-2018 (source), which comes out to 32 million people (assumes US adult population of 250 million).
Assuming AXS-05 is priced $1500/year ($125/month), which is towards the low end of well-known brand name antidepressants (source), Axsome would achieve $1.5 billion in sales with only 5% market penetration.
To assign a multiple to sales, we look at other early-commercial stage biotechs that have achieved at least 5% market penetration in their respective indications, specifically Harmony Biosciences (8.5x trailing sales) and Biohaven Pharmaceutical (12.7x). An example of a company later in the business cycle, Jazz Pharmaceuticals, trades at 3.2x trailing sales. To be overly conservative, we apply a 4x multiple on AXS-05’s projected $1.5 billion in sales gives us $6 billion in market cap, or $159/share.
In estimating a timeframe for AXS-05 to achieve 5% market penetration, we assume approval of AXS-05 in June 2022, and that the sales team currently on standby can be in full swing by the start of the 4Q22. Armed with the Digital-Centric Commercialization platform, the utility of which could be a key factor in AXS-05’s launch, and selling into a large, well-established market with inadequate current treatment options, we believe AXS-05 could ramp to achieve 5% penetration within three or four quarters, which takes us to the 2H23.
In summary, a 4x multiple on $1.5 billion in sales gives us our $159/share target price, or 400% return over Friday’s closing price, which we believe is achievable by the 2H23. We also note that AXS-05’s profile could allow it to eventually capture 10%+ of the 20 million patient-MDD market.
Comparative Analysis
While projecting AXS-05 market penetration and applying a sales multiple is more grounded in fundamentals, a comparison to early-commercial stage biotech Harmony Biosciences (HRMY) provides an interesting alternative perspective.
Harmony in-licensed pitolisant (Wakix; approved in late 2019) in 2019, which it markets for the treatment of excessive daytime sleepiness (EDS) and cataplexy in narcolepsy patients. According to Harmony, the diagnosed US narcolepsy patient population is only 72,000. In its FY21 earnings, Harmony reported an average of 3,800 patients on Wakix, which, coincidentally, represents just above 5% of the patient population. Using the smaller 44,000 of patients currently being treated, Wakix has already achieved 8.6% penetration.
Harmony reaching 5% (or 8.6%) market penetration in its second year on the market may or may not be relevant to estimating Axsome’s potential launch pace given the difference in patient population size, though it is conceivable that an orphan indication like narcolepsy would present challenges in actually reaching patients and prescribers which would not exist in MDD. Additionally, there must be pushback from at least some narcolepsy prescribers to a $42,000/year drug with similar efficacy to dirt-cheap generics.
Nevertheless, Wakix achieved 5% penetration and treated 3,800 patients, which generated (a rather impressive) $305 million of sales in 2021, driven in large part by Wakix’s $3,500/month price tag ($42,000/year). Harmony currently trades at a $2.7 billion valuation, or 8.5x trailing-twelve-month sales (includes 1Q22 results).
Tying this all back to Axsome, Harmony Biosciences, which 1) is addressing a patient population of only 72,000, 2) has no other clinical programs, save for a few tiny adjacent indications, 3) has already achieved 8.6% market penetration, limiting growth potential, and 4) charges $42,000/year for a drug that has similar efficacy to generics, is currently trading at 8.5x sales and a $2.7 billion dollar valuation.
If Axsome were to receive an 8.5x multiple on our projection of $1.5 billion in sales it would be valued at $12.8 billion, or $337/share, which would represent 10x the current price. Of course, using Harmony’s (seemingly inane) valuation to arrive at a price target for Axsome would be foolish. But we can also remember that AXS-05 has the potential to capture much more than our projected 5% of the MDD market, and that we’ve completely disregarded Sunosi, AXS-07, AXS-05 in Alzheimer’s agitation, and all of Axsome’s other programs in this analysis.
Risks
The key factor for Axsome over the next 12-18 months will be its ability to execute on the commercialization of multiple large-indication drugs at once (AXS-05, Sunosi, AXS-07). SG&A expenses will be in focus, especially with Axsome’s stated intention to bring on the entire Sunosi sales team from Jazz. The ability of the Digital-Centric Commercialization platform to reach prescribers will be in focus -- information on the platform has been scant. Axsome’s ability to capitalize on the prescriber overlap between many of its indications will also be important. Also, the company will need to demonstrate its ability to continue to progress its clinical pipeline in the midst of a broad commercialization effort, preferably avoiding continued regulatory delays caused by minor CMC deficiencies.
Aside from the business risks, investors should keep in mind that approval of AXS-05 may not mean significant share price appreciation; shares finished the week up only 20% following the postmarketing commitment news. So, either 1) near-certain approval AXS-05 was already mostly priced in, 2) people did not take the news as implying near-certain approval, or 3) the market either barely noticed or mostly did not care about AXS-05's near-certain approval update. While the market will have to care upon revenue and profit generation, investors may not get the reactions to news they expect in the meantime.
A Special Situation
In summary, Axsome’s stock, at a $1.2 billion valuation, is trading vastly below its fair value. As an increasing number of investors became disaffected along the long and winding road of regulatory delay, Axsome’s stock ceased to trade on fundamentals. It trades on news and technicals, muddled by conditioned skepticism and utter exhaustion. As a result, Axsome currently presents a rare opportunity for investors focused on sober fundamental valuation.
Strategy
We have opted to play Axsome via out-of-the-money (OTM) call options at various strike prices and expirations. In selecting strike prices, it may be useful to note that Axsome traded up to $70 leading up to AXS-05's original PDUFA date in August 2021, and that it spent the majority of 2020 between $65 and $85. However, as noted in the Risks section, the market has adopted a strong "wait-and-see" attitude towards Axsome since then.
The May expiration is unattractive due to its low-ish chance of capturing an FDA decision on AXS-05 (which may be ignored by the market anyway). The June expiration is only moderately less speculative, but offers a cheap way for investors to (most likely) gain exposure to an AXS-05 decision (if they believe it will move the needle). A third of our Axsome position is in June expiration calls.
The longer-dated expirations (September, December, and January ‘23) are trading near all-time lows after the AXS-07 delay news. The remainder of our Axsome position is split between the September and January ‘23 expirations; the December expiration has relatively little activity. Of particular interest are the further-OTM January ‘23 calls, like the $60 strike price (trading at about $5/contract), as they give time for Axsome to give informed guidance and preliminary numbers on AXS-05's commercialization, as well as navigate the AXS-07 delay and develop Sunosi.