Annovis: Imminent Alzheimer's Data Difficult to Handicap; Optimistic Overall
Given our optimistic view on Phase 3 Parkinson's data, we are inclined to expect some positive signs in Alzheimer's.
Overview
Annovis Bio is expected to report topline data from its Phase 2/3 trial in Alzheimer’s disease (AD) this week (management guidance was for April). It will mark the first time Annovis has reported new clinical data since 2021, when its Phase 2a data caused the stock to spike violently above $100/share before cratering back to $30/share. As we discussed in our initiation, we think the Phase 2a data in 2021, while inherently limited (e.g. small sample size, one month treatment duration), served as partial validation of buntanetap’s highly unique and intriguing mechanism of action.
Buntanetap is the only compound we are aware of that has demonstrated the ability to inhibit the production of multiple neurodegeneration-associated proteins, including amyloid precursor protein (APP; precursor to Alzheimer’s-associated amyloid beta), alpha synuclein (Parkinson’s), TDP-43 (ALS), and huntingtin (Huntington’s disease). Annovis terms these proteins “neurotoxic”. Uniquely, buntanetap enacts its downregulation of these proteins at the transcription level by interfering with mRNA. In total, we believe buntanetap’s mechanism is elegant and currently represents the best chance at a truly disease-modifying treatment in the neurodegenerative disease space.
Our most recent piece focused on handicapping Annovis’ Phase 3 Parkinson’s disease (PD) trial, which was originally expected to be the first of the two trials to report data (originally scheduled January), but has been delayed as the company investigates irregularities that were found in the blood samples of a small minority of patients. We explained that we are rather optimistic about the PD data, partially bolstered by our analysis of online patient anecdotes.
As compared to the Parkinson’s trial, the Alzheimer’s trial presents unique concerns/considerations, including:
A shorter treatment duration (3 months vs. 6 months)
A more advanced patient population (mild-to-moderate AD vs. early PD)
A smaller sample size (~80 patients per arm in AD vs. ~150 per arm in PD)
Less Phase 2a data on Alzheimer's vs. Parkinson’s (14 patients vs. 54 patients)
A lack of any online patient anecdotes for AD patients (like we have in PD).
Additionally, we are also concerned on account of the fact that we believe the doses of buntanetap selected for both the AD and PD trials may be lower than optimal. Said another way: at the very least, we don’t believe there was enough evidence to confidently select the doses that were selected for either the AD or PD trials (ranging from 7.5mg to 30mg, lower than the up to 80mg tested in the Phase 2a).
As a result, we think the probability of successful results in Alzheimer’s are significantly lower than Parkinson’s, but are still cautiously optimistic based on the belief that buntanetap’s mechanism is well-suited for the treatment of multiple neurodegenerative diseases (including AD and PD). While there are multiple shades of gray that the Alzheimer’s data could take, we think there is a decent chance of intriguing/positive data in the highest dose cohort (30 mg), and that Annovis is worth holding into the AD data given it also has Phase 3 Parkinson’s data expected in the coming weeks/months.
Phase 2a Data
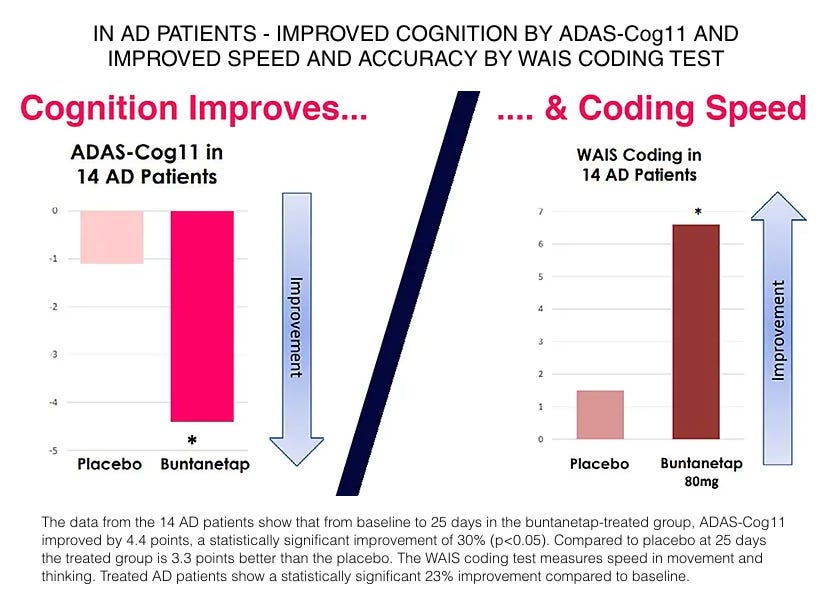
Recall that Annovis’ Phase 2a trial (reported in 2021) included 68 patients, but only 14 of those were Alzheimer’s patients (the remaining 54 were Parkinson’s). The results were rather impressive in terms of the absolute improvement on ADAS-Cog (4.4 point improvement vs. 1.1 for placebo), but have to be taken in context with the short treatment duration (one month) and very small sample size.
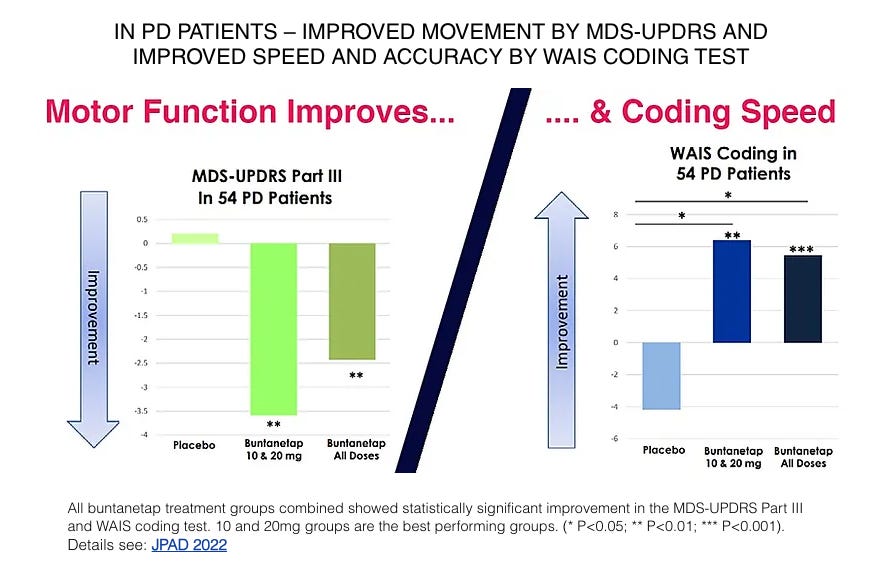
Annovis’s data in the 54 Parkinson’s patients was also impressive, delivering clinically relevant improvements in motor function (3+ point improvement in the best-performing doses). In both the Alzheimer’s and Parkinson’s patients, buntanetap improved scores on the WAIS Coding test, which is a digit-symbol coding test thought to be sensitive to small changes in function (link).
Taken in conjunction with buntanetap’s preclinical and mechanistic data, we argued that the Phase 2a data, while limited, was incrementally validating of buntanetap’s potential efficacy in multiple neurodegenerative diseases.
Dose
Notice that all of the 14 Alzheimer’s patients in the Phase 2a trial were dosed with 80mg daily. Despite the impressive Phase 2a Alzheimer’s results, Annovis decided based on the totality of data to select doses ranging from 7.5mg to 30mg daily for the AD and PD studies. It appears this decision was primarily based on the observation that the 10mg and 20mg doses performed best in the Parkinson’s portion of the trial (pictured below), as well as preclinical data which management believes suggests that milder downregulation of neurotoxic protein production may be favorable.
Given how small the Phase 2a data was (only 9 Parkinson’s patients per treatment arm), we think it may have been wise to select a wider range of doses for the Alzheimer’s Phase 2/3 and Parkinson’s Phase 3 trials. That said, the FDA apparently believed there was enough evidence to select 10mg and 20mg as the Phase 3 doses in Parkinson’s.
Buntanetap’s Mechanism
Buntanetap’s ability to downregulate the production of multiple neurodegeneration-associated aggregating proteins has been demonstrated in numerous animal studies (Teich, et al., 2018; Kuo, et al., 2019) as well as in humans (Galasko, et al., 2024). In the animal studies, buntanetap (formerly posiphen) has ameliorated mouse models of Alzheimer’s, Parkinson’s, ischemic stroke, and traumatic brain injury.
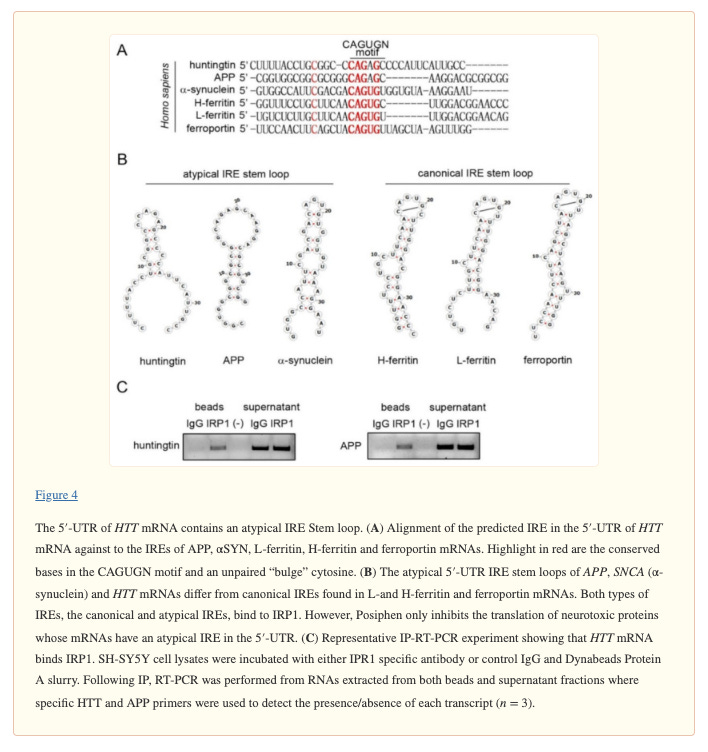
The ability to downregulate multiple neurodegeneration-associated proteins is attributable to buntanetap’s high binding affinity to a specific mRNA sequence that is shared among these proteins called an iron-response element (IRE). Interestingly, amyloid precursor protein (APP), alpha synuclein, TDP-43, and huntingtin—all of the proteins associated with the most-common and most-devastating neurodegenerative diseases—share an atypical stem loop in a their IRE, to which buntanetap binds (Chen, et al., 2021).
So why do these seemingly unrelated neurotoxic proteins contain near-identical IREs? Fascinatingly, buntanetap’s mechanism means that each of the neurodegeneration-associated proteins are regulated by intracellular iron status, which also points to the fact that these proteins have iron-related functions in healthy cells. For example, while all of its function have still not been elucidated, APP has been found to demonstrate ferroxidase activity (oxidizing iron from its unstable to its stable form, which can then be shuttled out of the cell). Interestingly, buntanetap has also been shown to not affect other iron-related proteins like the iron storage protein ferritin, which has a canonical rather than an atypical IRE in its mRNA.
Iron dysregulation, specifically intracellular iron accumulation (as a result of inflammation or other insults), leads to increased oxidation which results in a form of cell death called ferroptosis, which is extremely well-studied as a contributor to Alzheimer’s, Parkinson’s, and other degenerative diseases. It seems likely that the upregulation and eventual aggregation of amyloid beta, alpha synuclein, etc. are a result of higher intracellular iron levels, leading to a degenerative cascade.
For whatever reason, Annovis does not highlight the iron-modulating effect of buntanetap in many of its investor/marketing materials, nor the large body of literature on iron dysregulation in neurodegenerative diseases, perhaps out of simplicity.
Conclusion
Buntanetap appears to be well-positioned to address multiple aspects of the underlying pathology of neurodegenerative diseases, which leads us to apply some of the optimism that we have for Parkinson’s data to the Alzheimer’s data.
Still, given all that we have observed in the Alzheimer’s and broader neurodegenerative space, it is hard to believe that any company, let alone a little company with hardly any institutional ownership, could report groundbreaking, functional improvement in Alzheimer’s. We are eager to see the results and will be prepared to reevaluate our thoughts on the Parkinson’s data and buntanetap in general depending on the details.