Alpha Tau Medical: Localized Alpha Radiation, a New Paradigm in Oncology
(For smaller updates, commentary on news, positioning, etc., follow my X/Twitter)
Intro
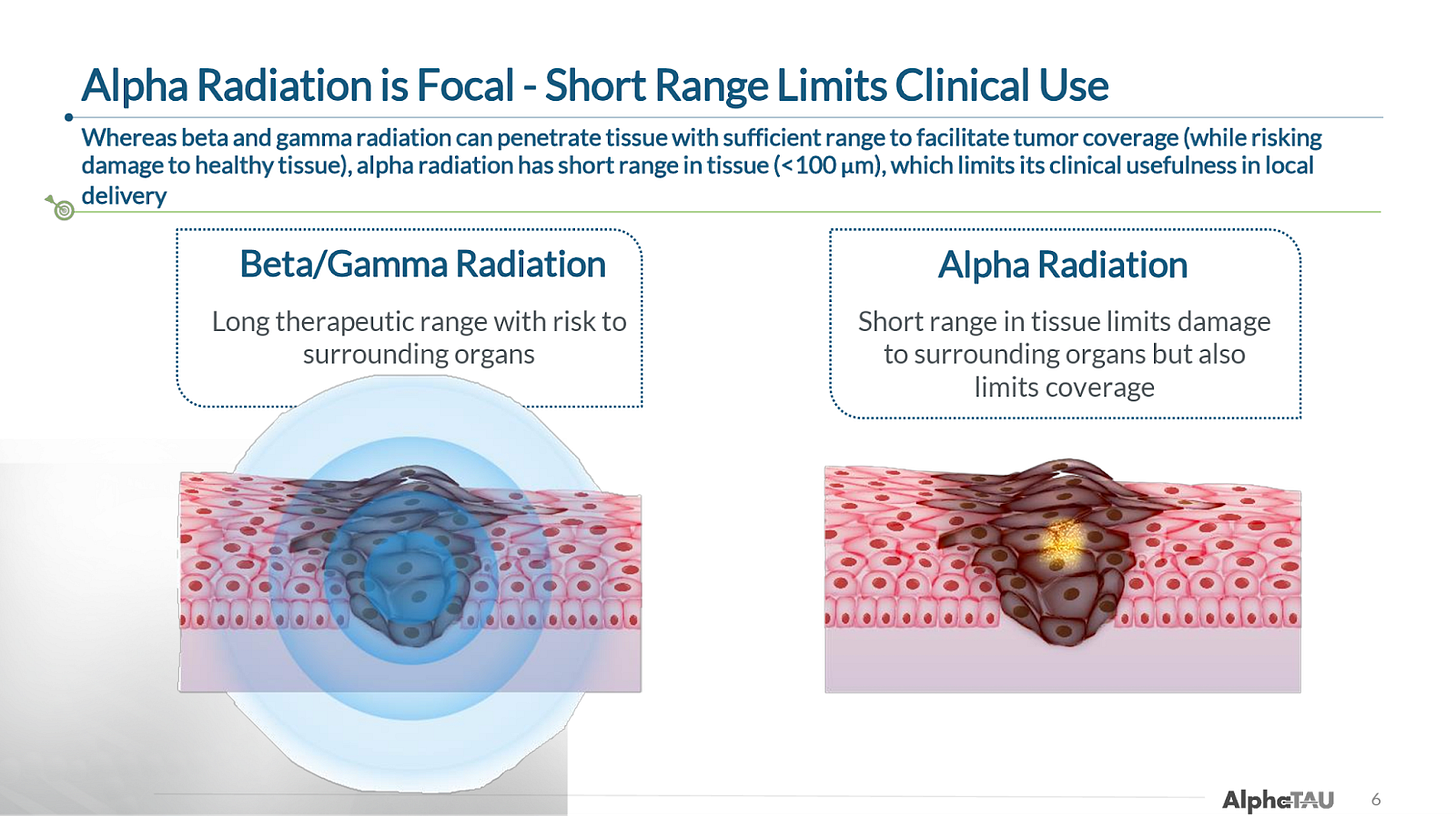
Alpha Tau Medical (DRTS) is pioneering the first and (as far as we are aware) only way of delivering alpha particle radiation locally to solid tumors. While the company is not currently well known, this represents a potentially significant breakthrough in oncology given that alpha radiation—which is significantly more effective at killing tumor cells than the beta, gamma, and X-ray radiation used in external beam radiation therapy (EBRT) and currently-approved radiopharmaceuticals—has historically been limited by its poor tissue penetrance and the risk of off-target damage.
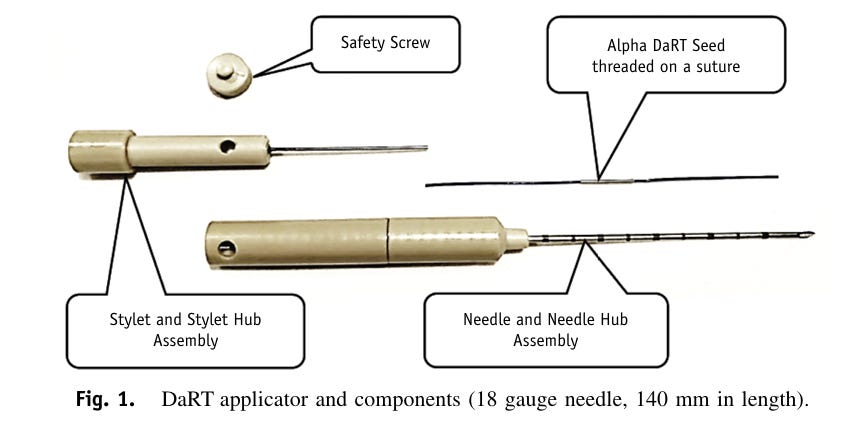
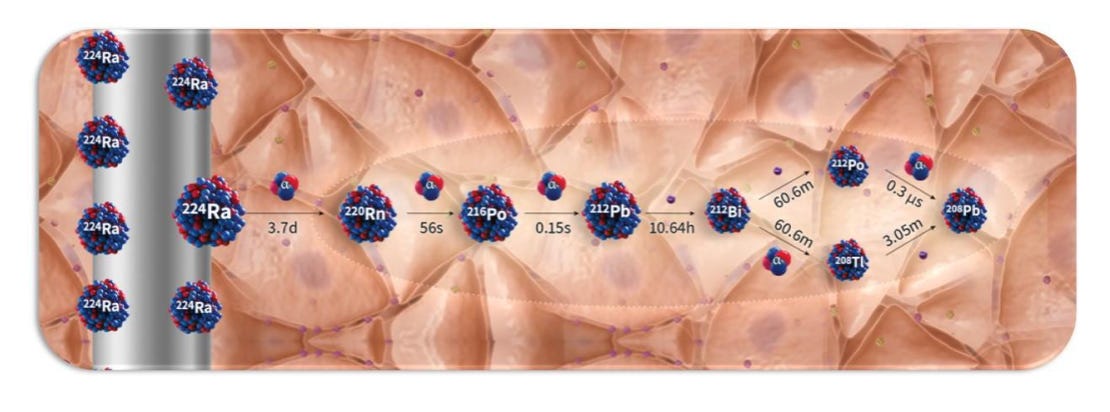
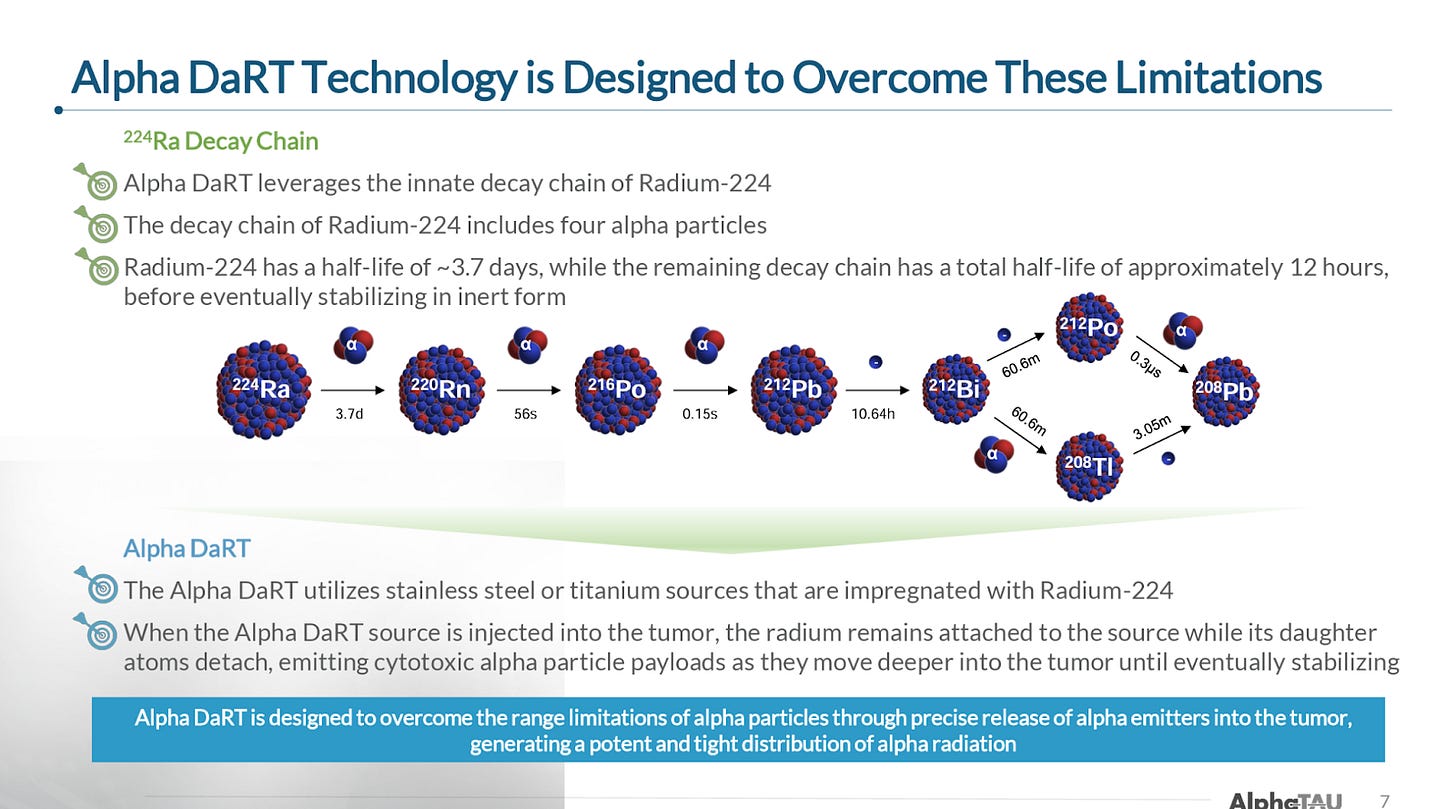
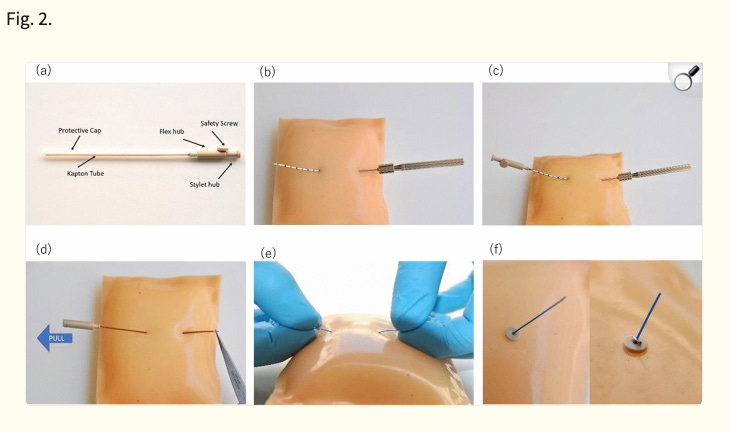
The company’s device and procedure, Alpha DaRT, involves the insertion of “sources” (stainless steel or titanium needles) that are coated with Radium-224 directly into the tumor. As the Radium-224 decays, it recoils off of the source, emitting alpha particles and penetrating deeper into tissue as it continues down its six-leg decay chain. This allows for 500-1,000x enhanced tissue penetration of the alpha radiation (vs. its natural penetrance), which so far has enabled the beginnings of what appear to be best-in-class efficacy (and safety) in multiple solid tumor types.
This efficacy, though scattered across a number of different trials (which in some cases are difficult to follow), includes:
100% complete response (CR) rate in cutaneous squamous cell carcinoma (cSCC)
100% objective response rate (ORR) and 50% CR rate in HNSCC in combo with pembro
97% disease control rate (DCR), 19% ORR, and at least one CR in pancreatic cancer
(Note: Some of these data are from very small sample sizes and/or exclusion of some patients)
We discuss these results in further detail below, including a comparison to SoC and leading clinical programs, but suffice it to say that these early data are very encouraging.
While cSCC is technically Alpha Tau’s lead indication with an expected pivotal readout of its US trial mid-2026, we (and we think investors taking an interest in the story) are becoming especially interested in pancreatic cancer, an extremely underserved and lethal cancer, where we note that the data reported to date, which is already more than doubling survival vs. SoC in some patient cohorts, employed only a partial application of Alpha DaRT onto the tumor—i.e. only 20-55% of tumors were covered with Alpha DaRT. With the 30-patient US pancreatic trial enrolling its first patient in early September and a topline readout expected in 3Q26, we think the results could turn out to be even better than the already impressive data to date.
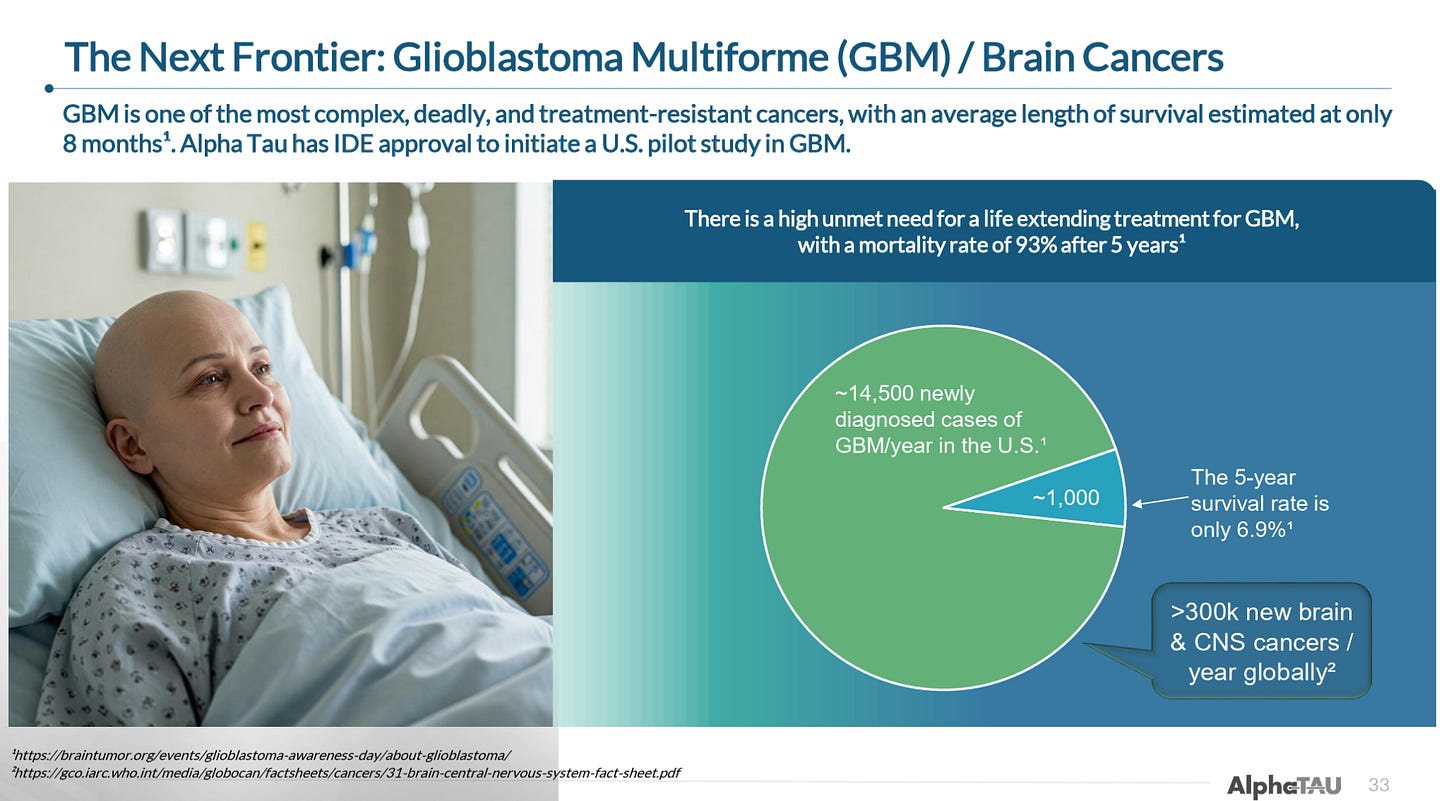
Beyond these primary indications, and with significant support from investigators both in its homeland Israel and internationally, Alpha DaRT is currently being trialed in a number of different cancers. Notably, Alpha DaRT is expected to enroll its first patient in a trial in glioblastoma multiforme (GBM), for which it has Breakthrough Device Designation, in the US by year end.
(Note: some of the timelines shown are out-of-date because this is an older slide deck—the company simplified the newer slide deck to only include its four leading indications)
Because the Alpha Tau story can be a bit complicated, and in the interest of presenting the story as clearly as possible, here are the key points in the Alpha Tau thesis:
Alpha DaRT represents the first localized, tumor-agnostic application of alpha particle-emitting radiation, which is far more effective than beta/gamma/X-ray radiation.
The only other viable application of alpha radiation to date has been radiopharmaceuticals, which are highly localized to cells expressing specific druggable target proteins, allowing for minimal off-target effects but also limiting application to a handful of cancer indications (e.g. prostate, GEP-NETs). These are still clinical-stage.
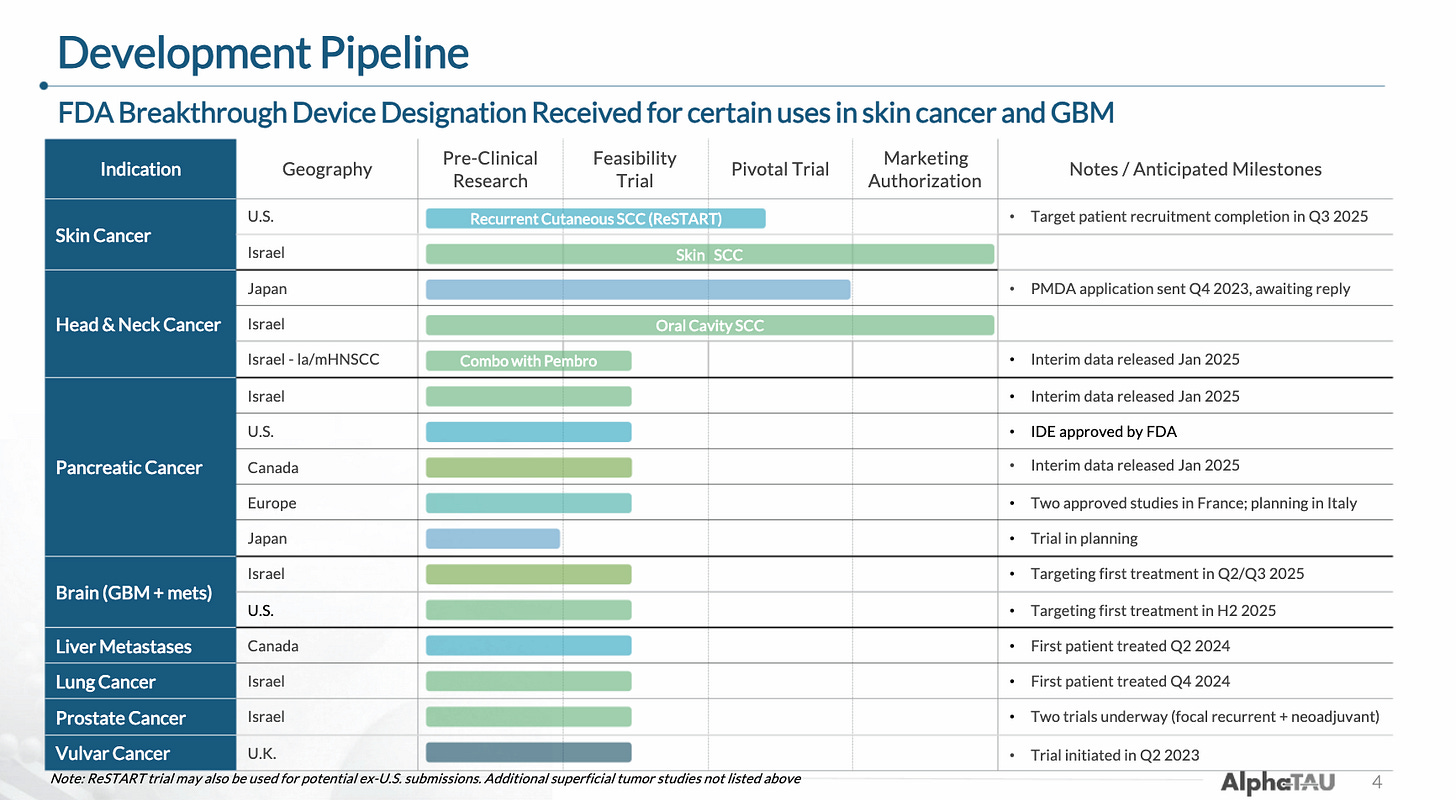
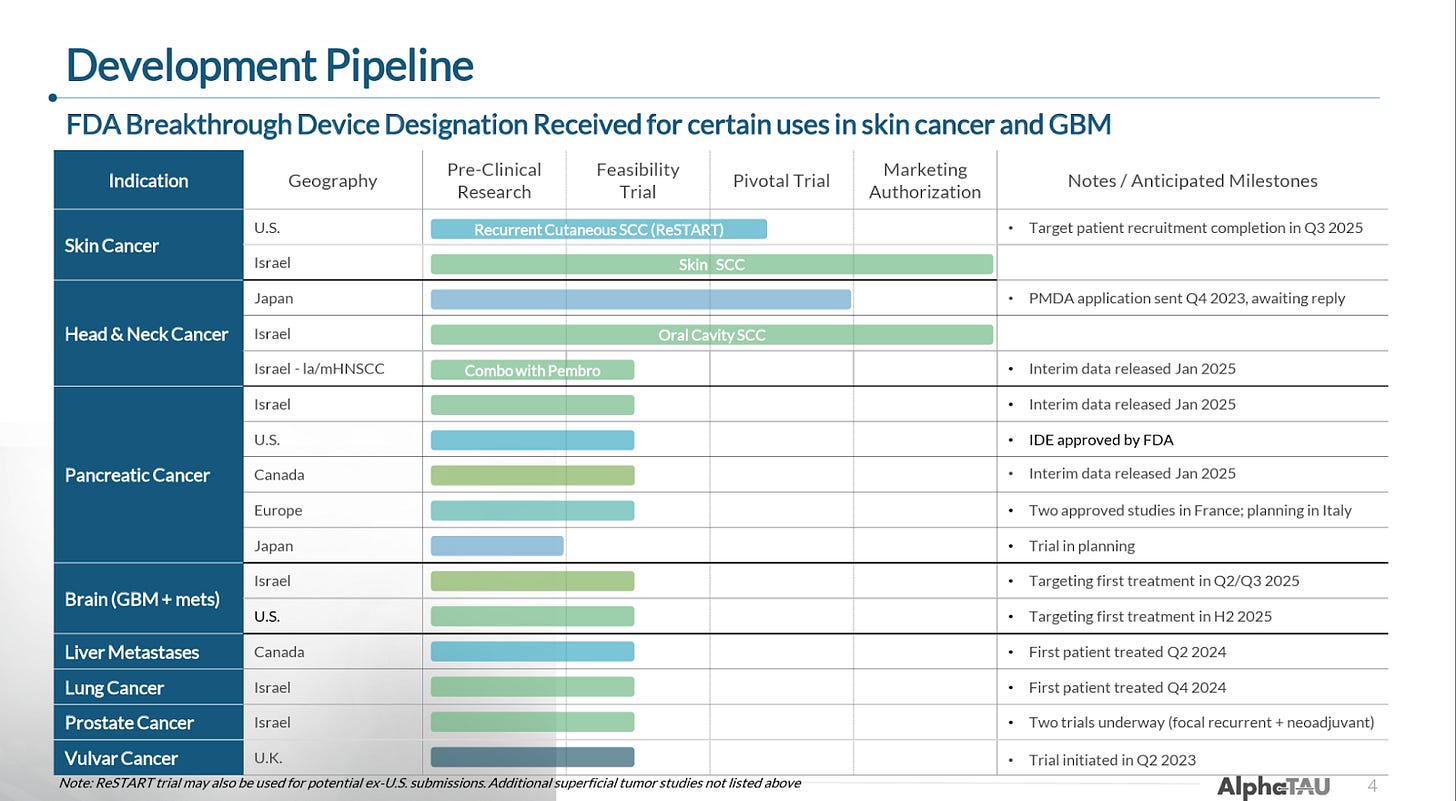
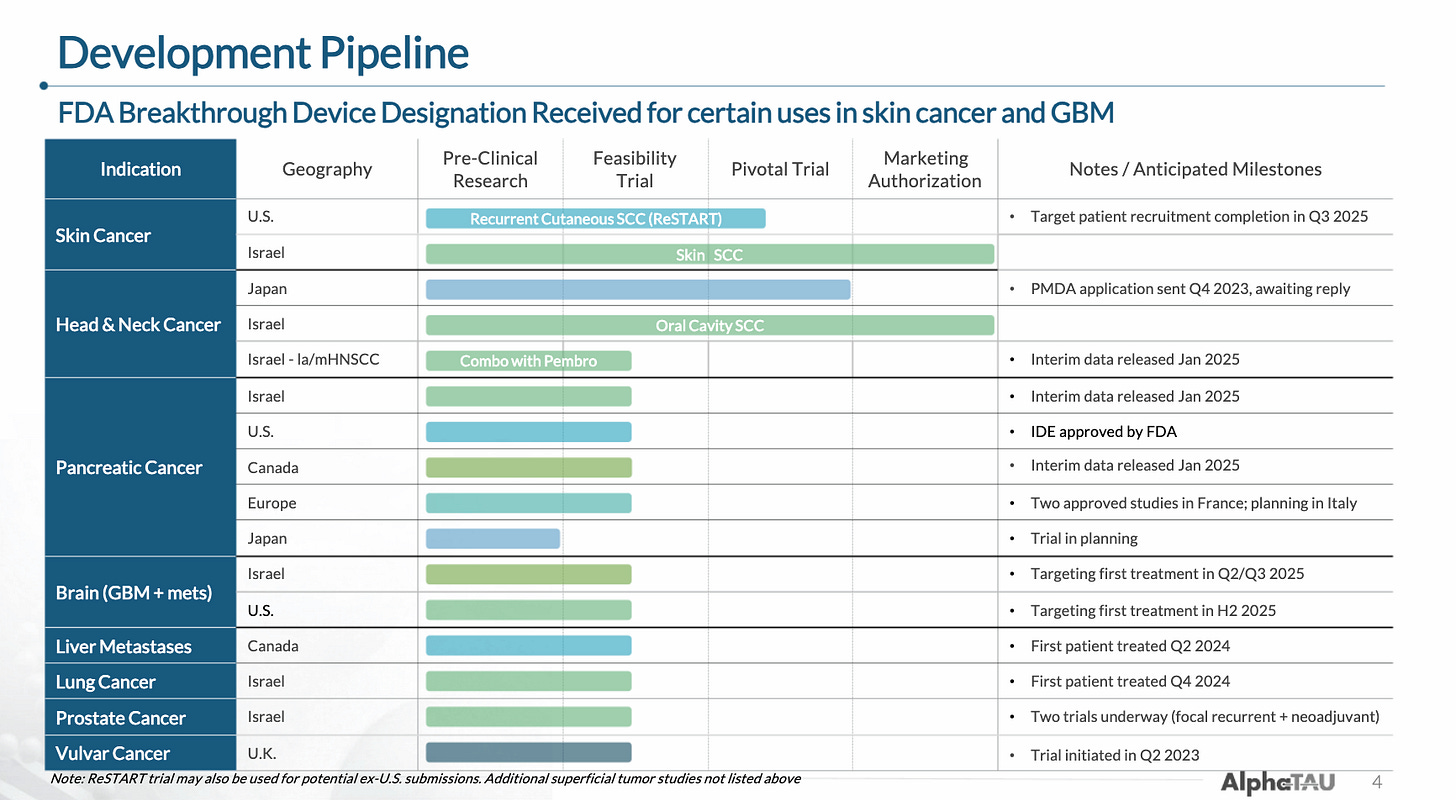
As a tumor-agnostic therapy, Alpha DaRT is currently being (or will imminently be) trialed in eight cancer types. The company has proven its ability to conduct multiple trials and has enrolled 100+ patients to date internationally.
Lead indication cSCC, which is already approved in Israel (but not commercialized), represents a proof-of-concept in a relatively non-lethal cancer, with a 100% CR and a pivotal top-line readout from US trial expected in mid-2026.
HNSCC (also already approved in Israel) has reported impressive data in combo with pembro, better than leading clinical-stage therapies, but is currently in a holding pattern as the company decides whether to start an expensive US checkpoint inhibitor (CPI) combination trial on its own or wait for a partner. Also currently awaiting a PMDA approval decision in Japan for HNSCC (expected by year end).
Pancreatic cancer, likely its most exciting program currently, has reported significantly better data vs. SoC despite employing only 20-55% tumor coverage, suggesting topline data from its 30-patient US trial expected in 3Q26 could show even greater efficacy.
GBM is expected to enroll its first patient in a 10-patient US trial before year end.
Multiple cases of shrinkage and resolution of distal/metastatic cancer lesions suggest Alpha DaRT’s activation of a systemic immune response.
Alpha DaRT’s extremely mild side effect profile and efficacy make it an attractive combination therapy, especially with checkpoint inhibitors (potential partnership opportunity).
Only burning ~$6 million per quarter; should have $75+ million of cash as of 9/30/25.
Despite these early indications of Alpha DaRT being a potential gamechanger in oncology and a broad clinical pipeline across numerous indications, Alpha Tau is currently trading at only ~$340 million of market capitalization.
Aside from being clearly undervalued on a simple future discounted cashflow basis (assuming approvals and eventual commercialization in multiple indications), Alpha Tau’s valuation looks especially low in light of recent business development and trading activity, including:
A flurry of acquisitions in late 2023 to 2024 of alpha radiopharmaceuticals companies, including Eli Lilly’s acquisition of POINT Biopharma for $1.4 billion in October 2023, BMS’s acquisition of RayzeBio for $4.1 billion in December 2023, and AstraZeneca’s acquisition of Fusion Pharmaceuticals for $2.4 billion in June 2024. All of these radiopharmaceuticals companies are currently going after the same few cancer types.
Nanobiotix (NBTX): Provides a “radioenhancer” that makes external radiation therapy more effective. Still has all the downsides of external radiation (e.g. toxicity, healthy tissue damage) and has reported lower efficacy than Alpha DaRT’s early data in their overlapping indications (primarily HNSCC). Currently trading at $1.3 billion of market cap after a recent rally fueled by data updates.
Merus (MRUS): Recently announced it will be acquired by Genmab for $8 billion, primarily for its HNSCC program which reported impressive data earlier in the year.
Alpha Tau appears to be entering a critical period during which it will go from being (or being perceived as) a small, relatively unknown, mostly Israeli-based oncology biotech, to a unique, paradigm-changing oncology company with multiple ongoing, potentially high-profile US trials. While it has taken some time for the story to come together, we think the next 12-24 months will be transformative for Alpha Tau and expect a concordant re-rating of its valuation during that time period.
Alpha DaRT
Alpha DaRT (Diffusing α-emitters Radiation Therapy) involves the injection of thin metallic seeds (tiny metal rods made of either steel or titanium) that have been coated with Radium-224 directly into tumors. After implantation, short-lived daughters of the Radium-224, such as Radon-220, Polonium-216, and Lead-212 recoil off of the metal seed and deeper into the tissue, suffusing the tumour with high-linear energy transfer (LET) alpha particles, while sparing most of the surrounding healthy tissue and avoiding systemic exposure. The seeds are either removed after 3-4 weeks or, in some cases, are left inside the body.
One of Alpha DaRT’s key innovations is the way that Alpha Tau traps the Radium-224 on the surface of the metallic seed, which allows the daughter particles to recoil off of the source/seed, allowing the isotopes to penetrate 4-5 mm into tissue, which is roughly 500-1,000x further than the ~40-90 microns that alpha-emitting isotopes normally penetrate through human tissue. The limited tissue penetrance of alpha particles has been one of the primary impediments to their utilization in radiation therapy, and why targeted alpha radiopharmaceuticals have been the first successful application of alpha particles (because they deliver the radioisotope directly to the desired cells).
This increased tissue penetrance allows Alpha DaRT unlocks the superior efficacy of alpha radiation, which, due to alpha particles’ greater mass and higher energy transfer rate, causes double-stranded DNA breaks, which potently trigger cell death. Beta radiation, by comparison, primarily causes single-stranded DNA breaks, which the cell can often repair.
In addition to enhanced damage through double stranded DNA breaks, there are a number of complementary mechanisms by which Alpha DaRT’s unlocking of alpha radiation creates such powerful efficacy and safety advantages over existing treatments. CMO Dr. Robert Den summarized these advantages in this video from early July:
Alpha DaRT’s localized application, limited tissue penetrance compared to other forms of radiation, and the highly potent nature of alpha radiation allows Alpha DaRT to administer a significantly higher level of radiation than what can be achieved with other radiotherapeutic modalities. In essence, EBRT (and other radiation therapies) are dose-limited by toxicity, while Alpha DaRT essentially removes this limitation.
The company has have found that the alpha particles don’t diffuse nearly as much through healthy tissue as they do through cancerous tissue (because of the disorganized nature of cancerous tissue), which further limits the damage caused to surrounding tissue.
Because DaRT is administered directly inside the tumor and spares healthy tissue, it avoids killing the T cell and B cell suites that are usually sitting around the periphery of the tumor, which are important for killing the tumor cells.
The cell destruction caused by alpha radiation activates the surrounding immune cells, creating immune activation towards tumors, i.e. making a cold tumor hot.
The recognition of these leaked tumor-specific proteins and markers by the immune system leads to a systemic response that allows the immune system to detect and defeat distant lesions, called an abscopal effect.
Alpha DaRT’s true innovation is allowing for intratumoral delivery of alpha radiation therapy, which to our knowledge is completely unique in the oncology space.
Pipeline
While Alpha DaRT’s mechanism is applicable to essentially any solid tumor type, the company originally focused on external/superficial tumor types like cSCC and HNSCC due to the practical advantages and the ability to easily monitor improvements. More recently, Alpha Tau began expanding into cancers with severe unmet need like pancreatic and glioblastoma.
The company’s pipeline includes eight indications for which it has either completed, begun, or is about to begin trials (some timelines in the below image are out-of-date).
In total, the company has 24 trials that are either actively recruiting or expected to begin recruiting soon according to ClinicalTrials.gov, many of which are investigator-initiated.
Skin Cancer
In its most advanced indication, cutaneous squamous cell carcinoma (cSCC), Alpha DaRT is actually already approved in Israel (approved in 2020), though the company has not yet launched commercially as it is focusing its financial and operational resources on the US (where it will be able to set a higher global pricing precedent). While cSCC is less lethal than most cancers, the most common treatments like surgical resection and EBRT can be deformative and/or come with significant side effects.
The data Alpha DaRT has reported in cSCC to date, its largest in any indication, suggest it could provide an essentially curative treatment option with comparatively limited disfigurement and side effects.
The company is currently conducting a pivotal study in the US that it expects will readout data around 2Q26/mid-2026, with a submission for approval expected thereafter, though we note that the company may delay commercialization. We also note that Alpha DaRT was awarded Breakthrough Therapy Designation in June 2021 in cSCC.
Data To Date
28-Patient First-In-Human Trial
The first trial of Alpha DaRT in skin (and oral) SCC enrolled 28 patients who either failed or were unfit to receive definitive therapy (resection/EBRT). The trial reported:
100% ORR at six weeks post treatment
Including a 79% CR rate.
These results were enough to support the approval of Alpha DaRT for skin SCC in Israel.
This pooled analysis spanned 81 tumors in 71 patients across patients with recurrent or unresectable cSCC, HNSCC, or basal cell carcinoma (BCC). As stated above, it includes the 28-patient initial pilot study we just discussed. Across the 71 patients, Alpha DaRT delivered:
99% ORR
Including an 89% CR rate
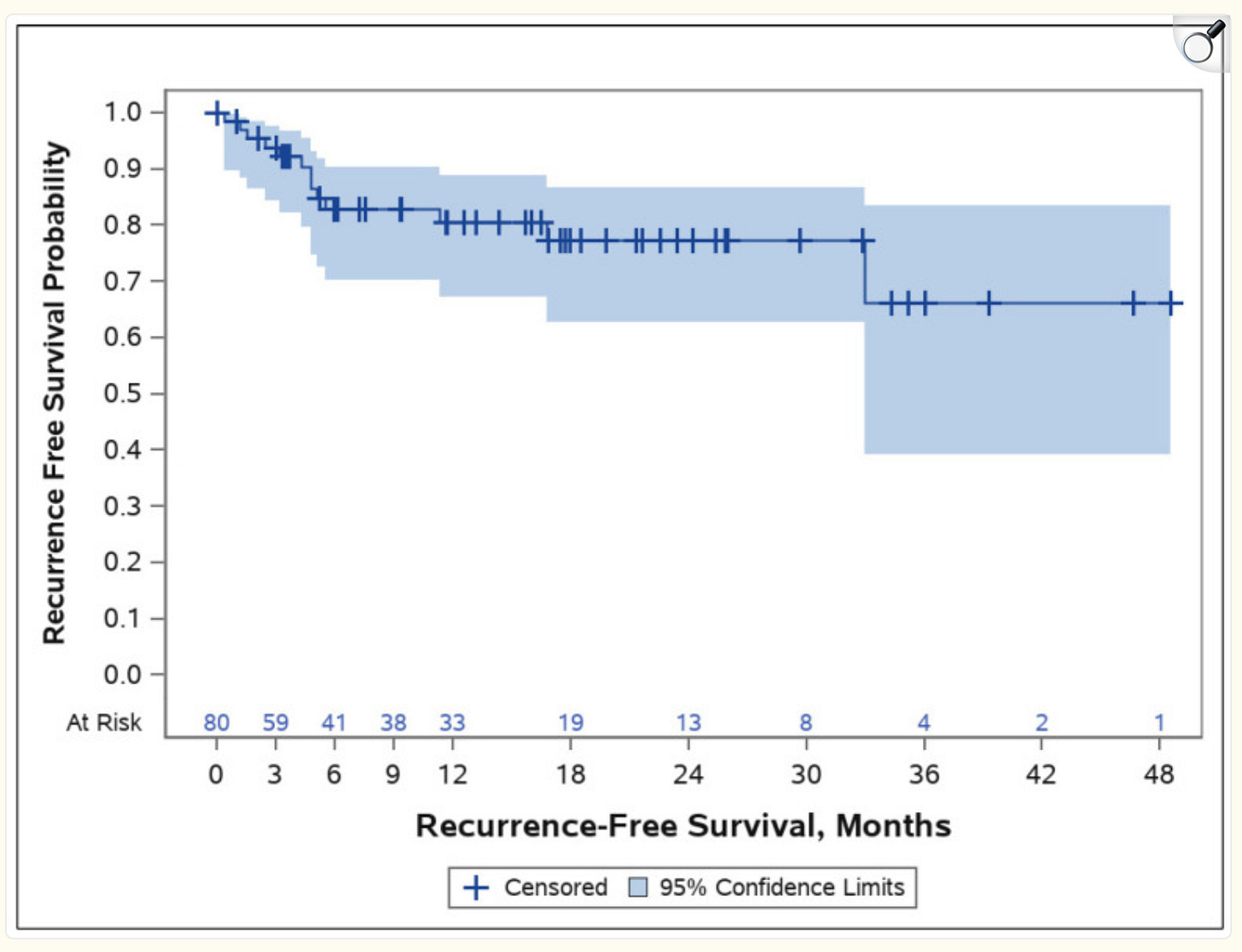
The two-year actuarial local recurrence-free survival (essentially two-year PFS) was 77% (shown below), and if you look at the recurrence/survival plot below, you can also see that the 4-year PFS is around 65%. These results would have been even better if you were to exclude the 28-patient pilot trial, which had had lower efficacy and durability than subsequent trials as the company initially prioritized safety and optimized the Alpha DaRT device/procedure.
As for safety, 27% of patients developed acute grade 2 or higher toxicities, all of which were resolved with conservative measures.
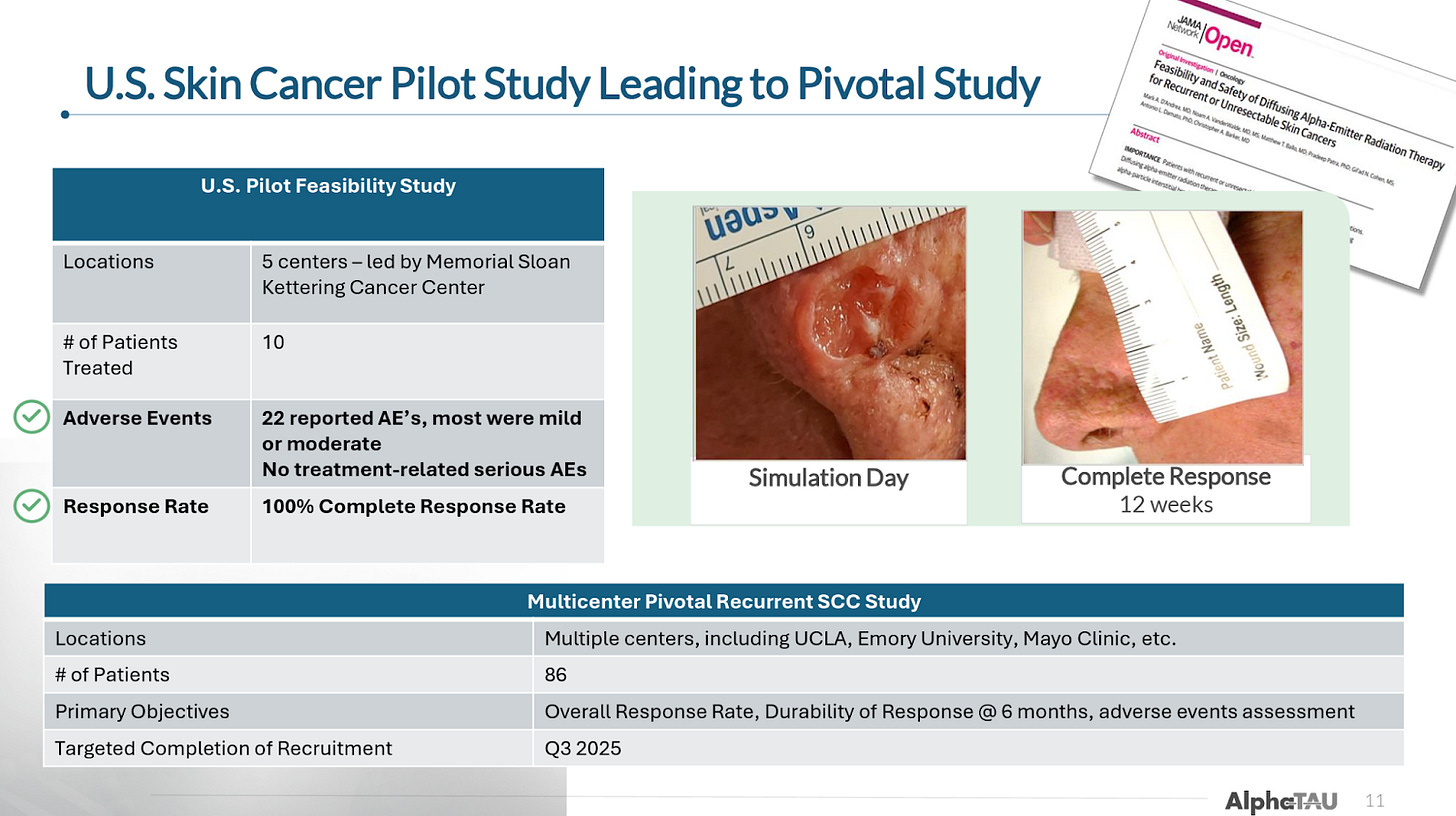
Memorial Sloan Kettering led a US pilot trial of 10 patients with skin SCC that was published in JAMA. These patients had either failed prior radiation therapy (60%) or had a tumor that was deemed unresectable (40%).
All 10 patients (100%) achieved complete response.
It is worth noting that all of these results were achieved without combination with immunotherapy or chemotherapy, some combination of which would likely be utilized for at least some patients with more challenging diseases in a real-world setting.
Market and Treatment Landscape
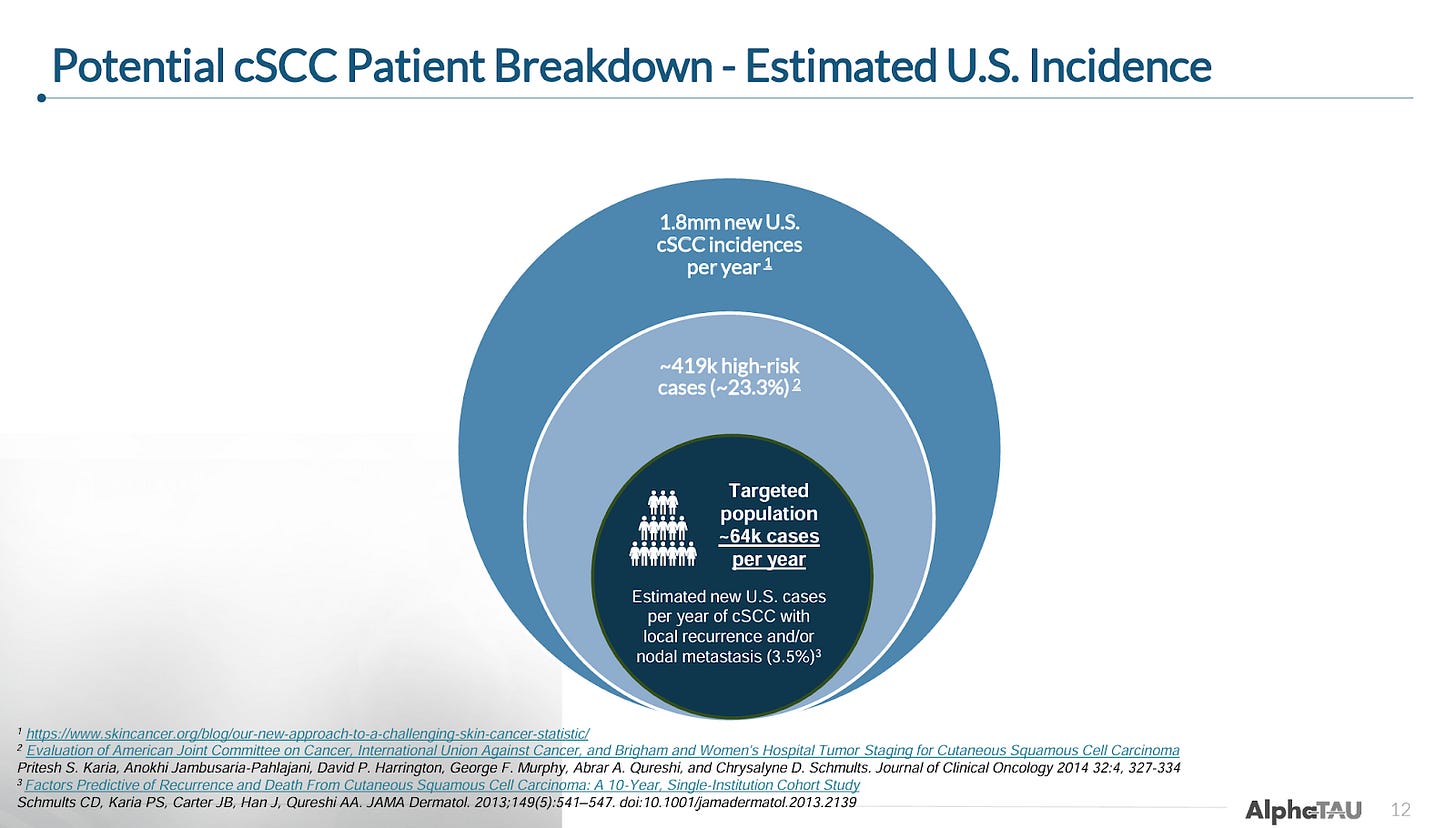
Despite relatively high response rates seen with surgical resection (e.g. Mohs; used in the majority of patients), non-surgical local treatments (e.g. cryotherapy, photodynamic therapy; used in very early-stage disease), and EBRT (used alone and in combination with surgery or therapeutics), a fair number of patients are either ineligible for or fail these treatments. Alpha Tau will target the approximately 64k patients in the US that have tumor recurrence after surgery or EBRT and patients with local metastasis.
This cohort of “advanced” cSCC patients is significantly underserved by existing therapies. Treatment options primarily consist of checkpoint inhibitors, EGFR inhibitors, and chemotherapy.
Checkpoint inhibitors, the most efficacious (and durable) treatment for these patients, report:
ORRs ranging from 45-70% (Zhang et al., 2022; Hein et al. 2023)
CR rates ranging from 7-28% (Zhang et al., 2022; Hein et al. 2023)
Alpha DaRT so far appears to represent a dramatic improvement over current SoC efficacy in these patients.
Pivotal US Skin SCC Trial (ReSTART)
The company is currently conducting a pivotal trial in 86 patients with recurrent cSCC for which it expects to complete enrollment of ReSTART in 4Q25 (likely towards the latter half; previous guidance was 3Q25), with topline results expected in 2Q26/mid-2026. The company expects to submit for approval with the FDA shortly thereafter, which could support potential commercialization in 2027.
It is also worth mentioning that Alpha DaRT is also in two other cSCC trials:
An 80-patient investigator-initiated study in France in different malignant cutaneous tumors (mostly SCC and BCC) which enrolled its first patient in 2022.
An investigator-initiated study led by Emory University in immunocompromised cSCC patients is expected to begin enrolling in the 2H25
While cSCC is Alpha Tau’s most advanced indication, we note that the company may not elect to commercialize in cSCC after receiving approval, primarily because cSCC is a relatively non-lethal cancer with good treatment options, which could require more resources to sell into. We think it is likely that the company could choose to prioritize its resources for advancement in pancreatic cancer, and to a lesser extent GBM.
Regardless of whether the company decides to pursue commercialization immediately upon approval or to wait, the cSCC data are a nice, visible, large(ish) sample size illustration of Alpha DaRT’s powerful potential on both efficacy and safety. We still expect the ReSTART readout in mid-2026 to be one of the larger near/medium-term catalysts for Alpha Tau.
Head and Neck Squamous Cell Carcinoma (HNSCC)
The company is also advancing Alpha DaRT in HNSCC, which is similar to cSCC (both affect squamous cells), with the distinction that HNSCC affects mucosal cells of the head and neck region, while cSCC affects epidermal cells in the skin. HNSCC is generally harder to treat, with lower rates of tumors eligible for resection, lower response rates to immunotherapy, and significantly higher rates of mortality, especially in the recurrent/metastatic setting.
In HNSCC, Alpha DaRT:
Has approval for oral cavity SCC (which encompasses most HNSCC cases) in Israel
Is awaiting a decision on its approval application in Japan (expected 2H25)
Reported early data from a Pembrolizumab (Keytruda) combination trial in January
While the data is much more limited than for cSCC, early indications again point to Alpha DaRT having potentially best-in-class efficacy when used in combination with a checkpoint inhibitor (current SoC).
11-Patient Pilot Study
These 11 patients had all received either prior external beam radiation or brachytherapy (i.e. had recurrent disease). The trial administered DaRT as monotherapy (no checkpoint inhibitor) and reported:
82% ORR
Including a 27% CR rate
DCR of 91%, meaning only one patient had progressive disease
Pembro Combination
The company also conducted a trial of Alpha DaRT in combination with pembrolizumab in Israel. This trial includes patients with either metastatic or locally advanced disease.
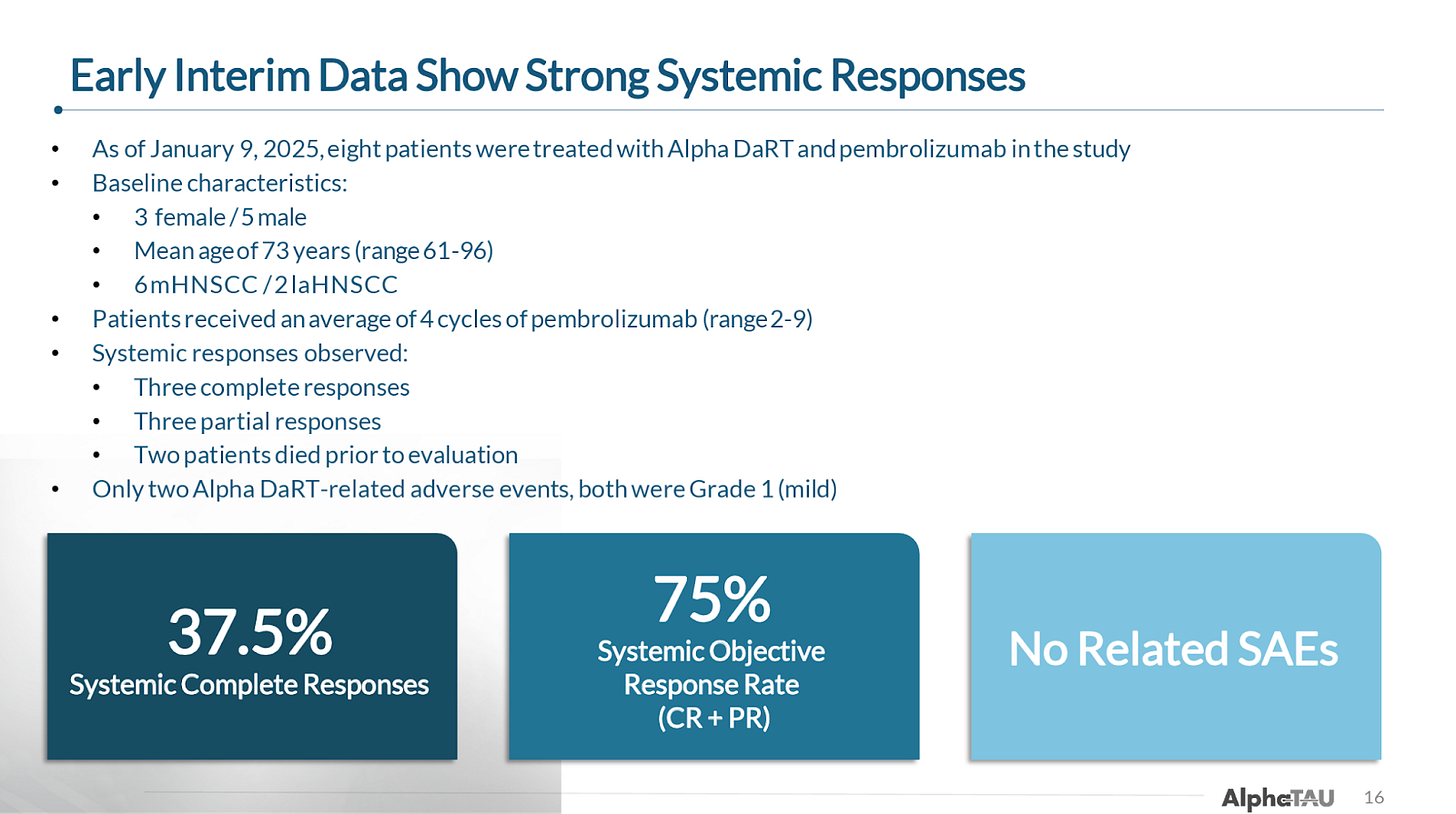
In January, the company reported interim results from the first 8 patients (6 metastatic; 2 locally advanced) in this trial, showing:
75% ORR
Including 38% CR rate
If you exclude the two patients that died prior to their first evaluation, the results improve to:
100% ORR
Including 50% CR rate
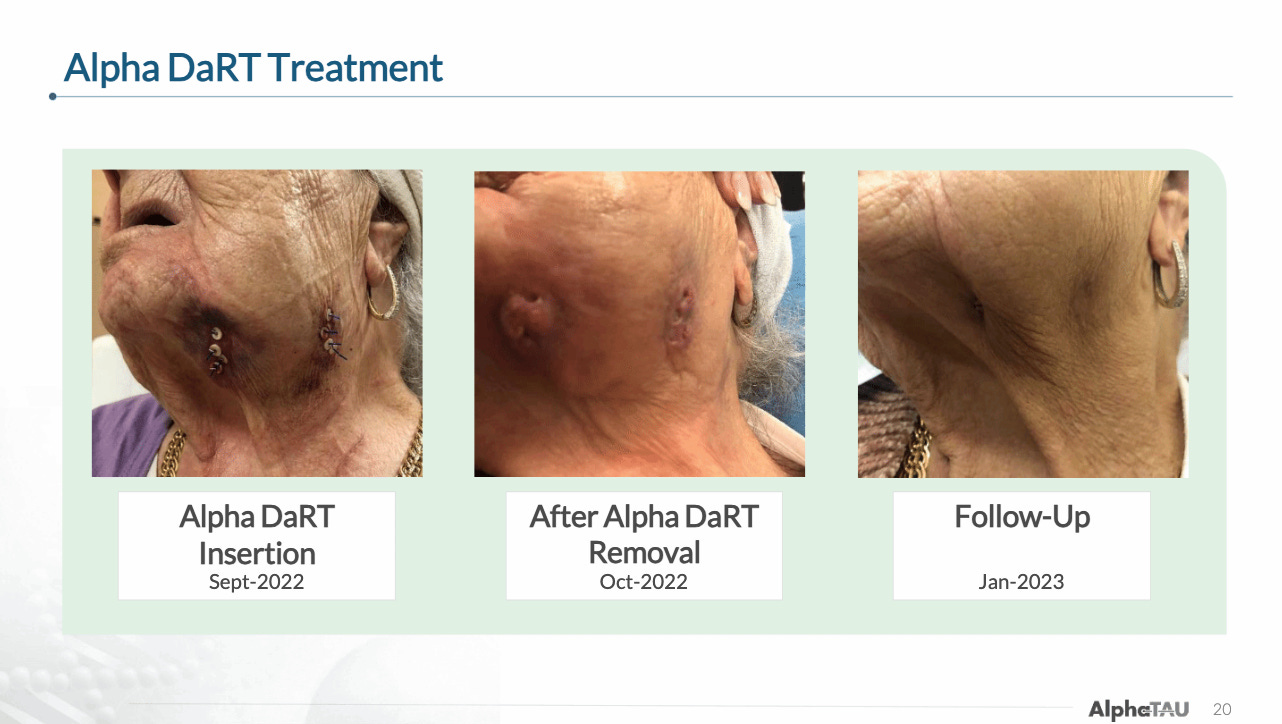
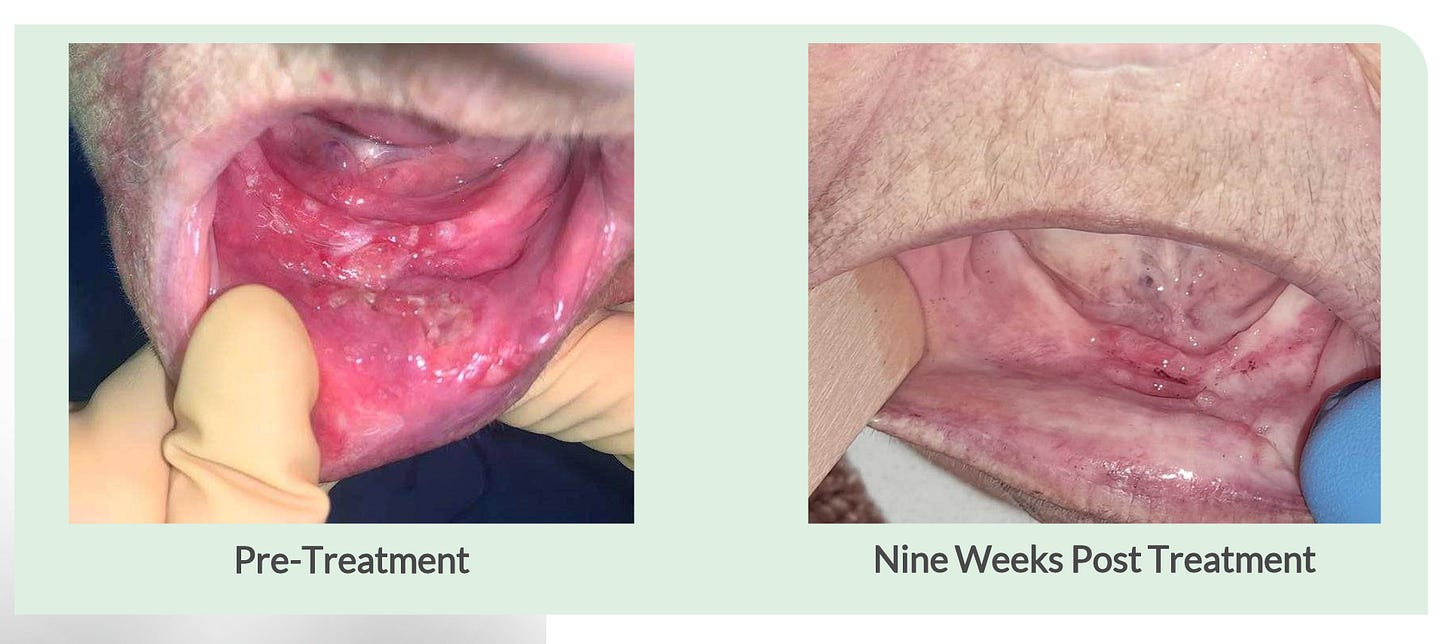
The company also provides an example of a patient from the trial that had was treated in 2022 and was still alive as of October 2024 follow up with no evidence of disease. You can see from the images below that the patient had lesions both in her neck as well as in her jaw, and while the Alpha DaRT treatment was only applied to the neck, she also experienced complete resolution of the lesions in her jaw, demonstrating the potential systemic effect that Alpha DaRT can deliver in combination with immunotherapy/.
While the sample size is quite small and we will await an update on the durability of these responses, the early efficacy signals are promising. Unfortunately, though this trial was designed to enroll up to 48 patients, an interim analysis deemed the trail successful in demonstrating Alpha DaRT’s proof of concept and enrollment was stopped shortly after the January data update.
As such, the company is in a bit of a holding period with regards to HNSCC, considering whether it should embark on an expensive checkpoint inhibitor combination trial in the US on its own or wait until it can potentially strike up a partnership with a larger checkpoint inhibitor pharma company. In the meantime, the approval decision expected from the Japanese PMDA before the end of the year could serve as an incremental catalyst for the stock, though we believe the company will likely continue to wait for US approval to commercialize.
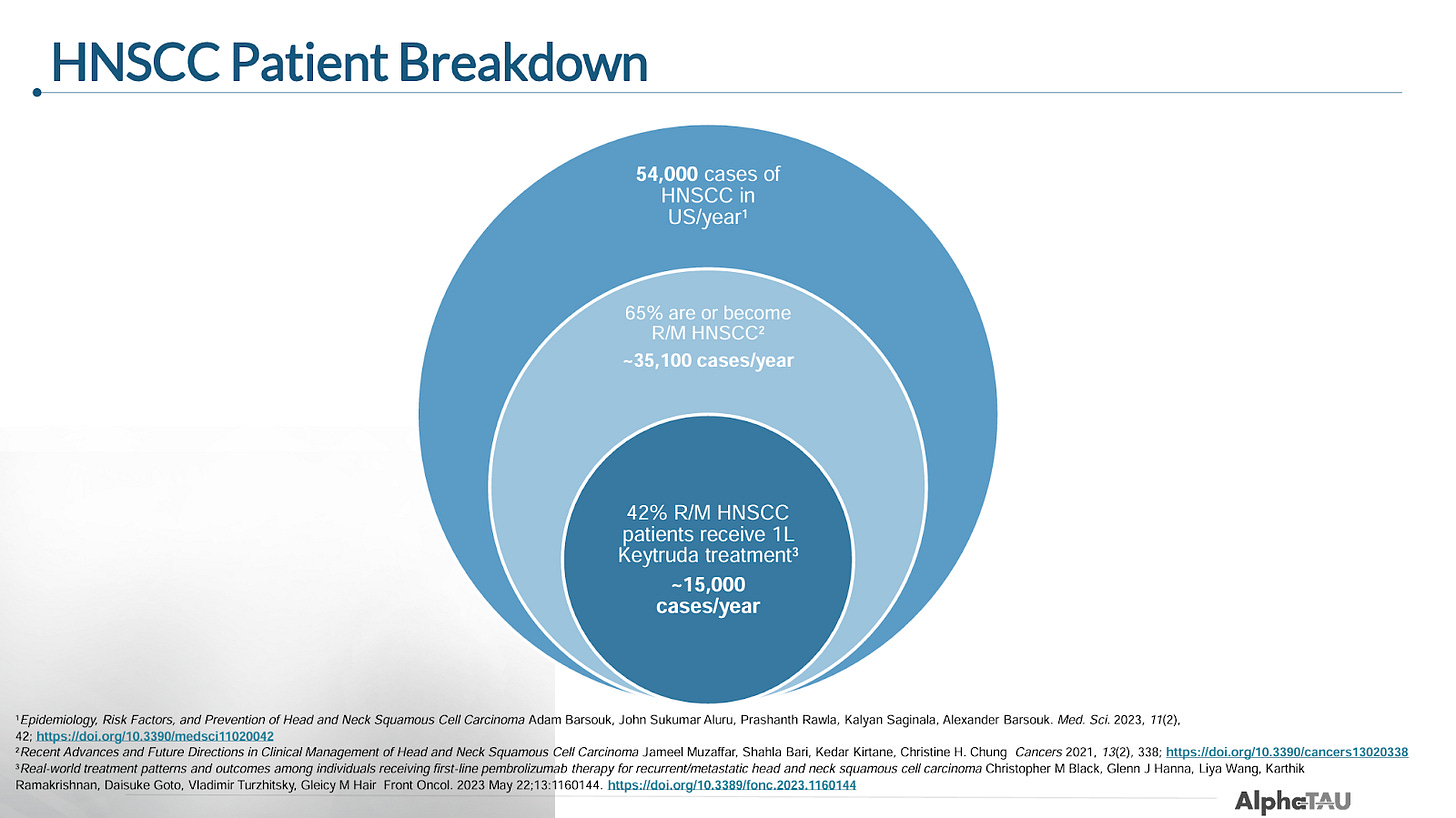
Market and SoC
Like most companies developing drugs for HNSCC, Alpha Tau is focusing on recurrent unresectable and metastatic patients (r/m HNSCC). The company estimates there are roughly 15k cases of R/M HNSCC per year in the US.
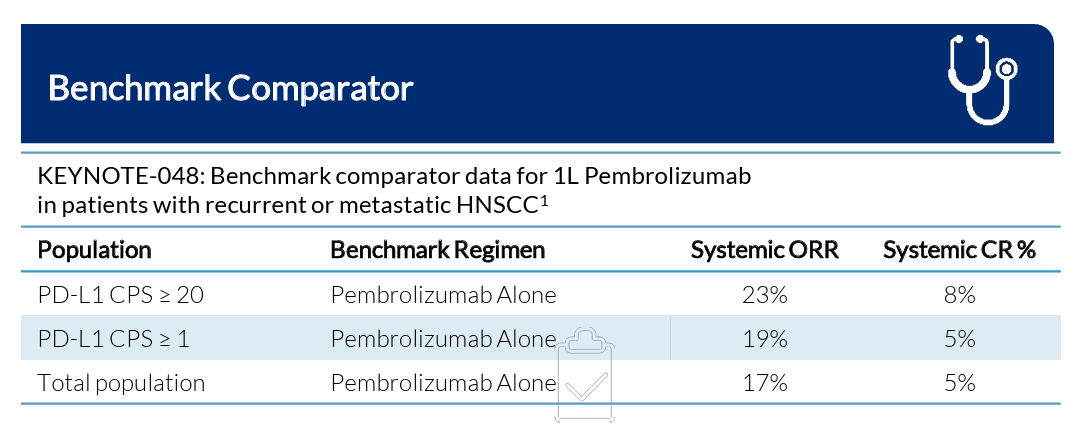
This is the same patient population that was investigated in pembrolizumab’s pivotal KEYNOTE-048 study that led to its approval in r/m HNSCC, where it currently serves as first-line standard of care treatment.
In the pivotal KEYNOTE-048 trial, pembrolizumab reported:
17-23% ORR
Including 5-8% CR
mOS of 14.9 months
The company believes that these pembrolizumab results, which so far Alpha DaRT appears to very handily beat, will serve as the benchmark to beat when it decides to initiate another study in HNSCC.
Clinical Development Landscape
With pembrolizumab and other available treatments leaving a high unmet need for patients, there are a number of companies advancing potential treatments in HNSCC, most of which are conducting trials in combination with pembrolizumab. Bicara Therapeutics and Merus are two of the most prominent in the space.
Bicara Therapeutics
Bicara is advancing ficerafusp alfa, a bifunctional antibody targeting EGFR and TGF-β. The company provided extended follow-up data from its 28-patient pembrolizumab combination Phase 1 trial at ASCO in June that showed:
54% ORR
Including 21% CR rate
mPFS 9.9 months
mOS 21.3 months
Bicara began a pivotal Phase 2/3 trial for ficerafusp alfa in combination with pembrolizumab in February.
Merus
Merus, which was just acquired by Genmab for $8 billion, is advancing petosemtamab, a bispecific antibody targeting EGFR and LGR5, both as a monotherapy in second-line R/M HNSCC and in combination with pembrolizumab in first-line R/M HNSCC. Petosemtamab has Breakthrough Therapy Designation in both indications.
In May, Merus reported updated efficacy data in combination with pembrolizumab in 45 PD-L1-positive R/M HNSCC patients showing:
63% ORR
Including 14% CR rate
mPFS of 9 months
mOS not reached; 79% at 12 months
While Merus is also pursuing colorectal and some other solid cancers, this HNSCC data was the primary driver of the recent acquisition by Genmab for $8 billion. This highlights the potential value of Alpha Tau in HNSCC alone given it has so far delivered better results than both Merus and Bicara, though data is still very early. We also note that Alpha DaRT, as a local and relatively safe therapy, could theoretically be used in combination with immunotherapies like Merus’s petosemtamab.
We would expect more clarity from the company regarding next steps in HNSCC in the coming months, as well as the potential for updated data from the now-halted Israeli trial.
Pancreatic Cancer
Pancreatic cancer seems to have become the most exciting indication for Alpha DaRT in 2025. Pancreatic cancer is the third-leading cause of cancer deaths in the US, with about 50% of cases already metastatic upon diagnosis (Stage IV) and up to 87% of patients have tumors that are not surgically resectable, either because of poor margins or metastatic disease. There are very few treatments available for unresectable and metastatic patients, essentially consisting solely of chemotherapy, with median survival of 5-8 months.
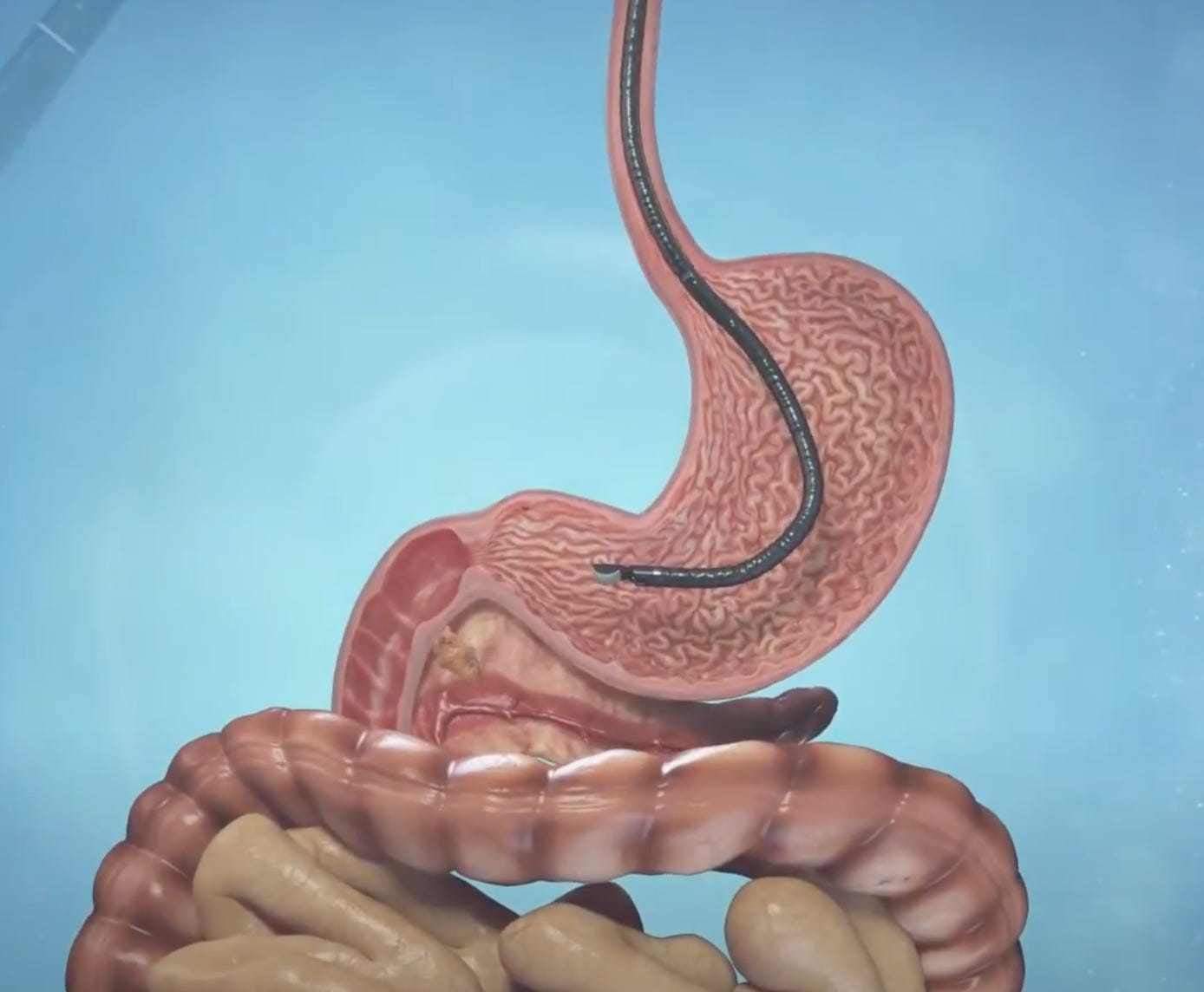
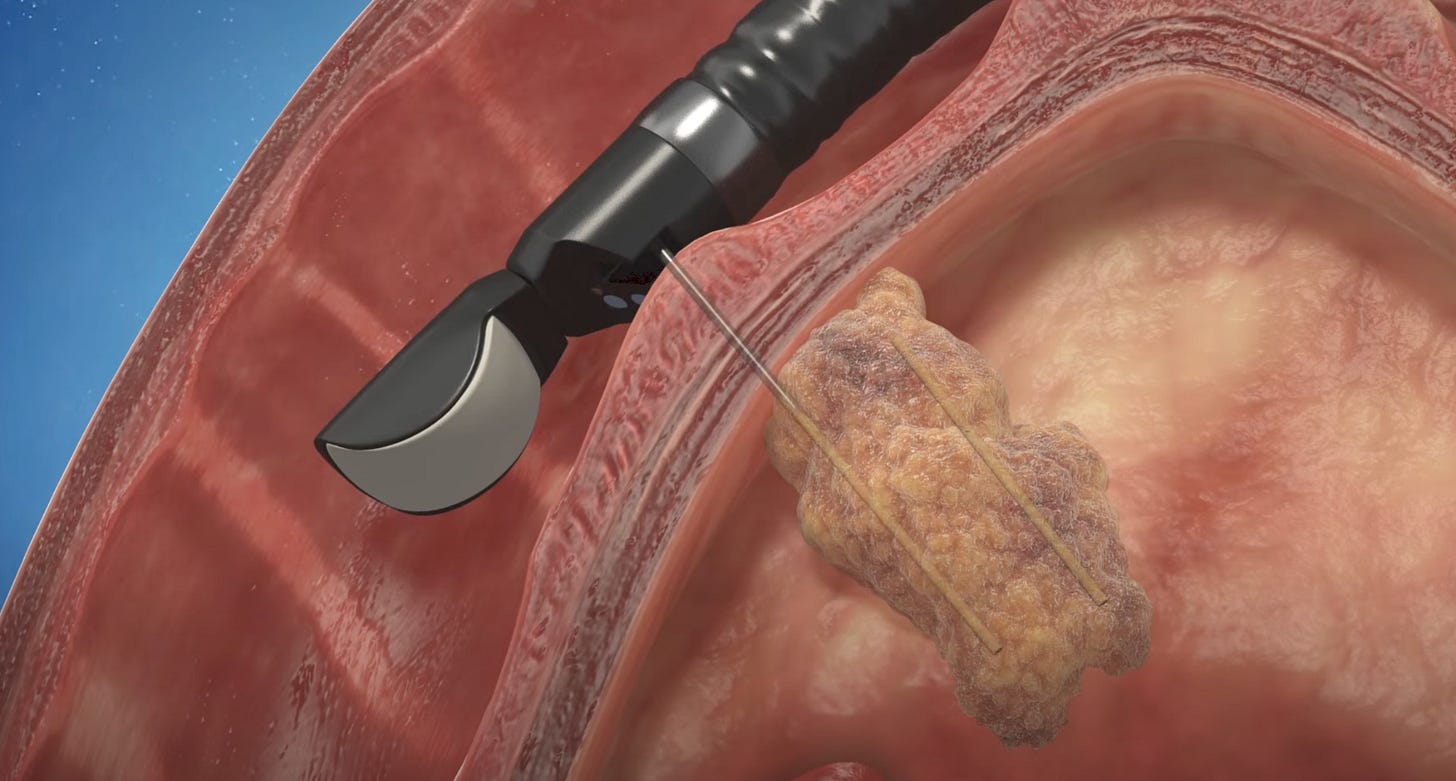
To adapt the Alpha DaRT treatment to an internal organ such as the pancreas, Alpha Tau piggybacked on an endoscopic diagnostic procedure that is already performed in pancreatic cancer patients called endoscopic fine needle aspiration (FNA). Alpha Tau created a device that allows for the loading of Alpha DaRT sources into the needle that is used during FNA, which allows the Alpha DaRT sources to be applied directly to the pancreatic tumor via ultrasound (video here).
In this interview, CMO Dr. Robert Den explains that this application of Alpha DaRT requires very little training for advanced endoscopists (an endoscopists specialized in diagnostic and therapeutic procedures). The procedure takes about 45 minutes once a physician is proficient, and patients are discharged from the hospital on the same day. During the January R&D day, Dr. Corey Miller, advanced endoscopist and assistant professor at McGill University, also testified to the ease of the procedure.
Data to Date
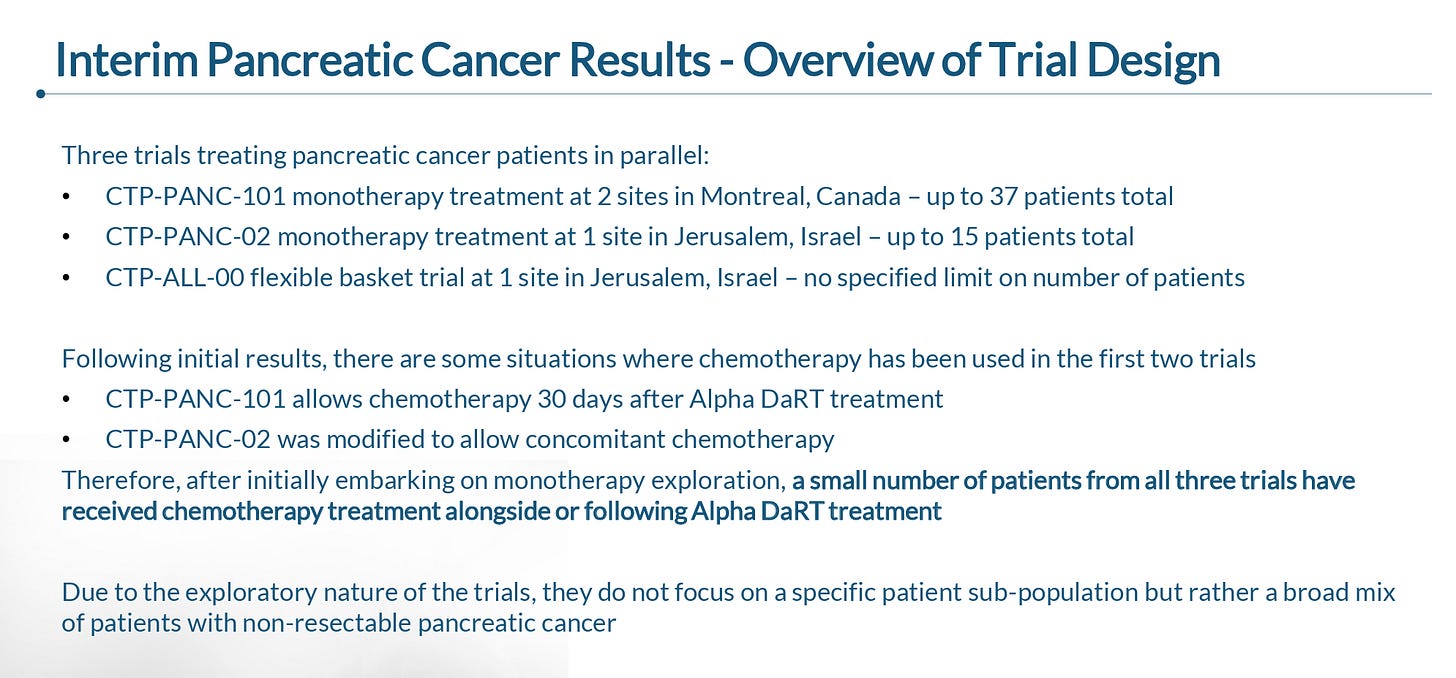
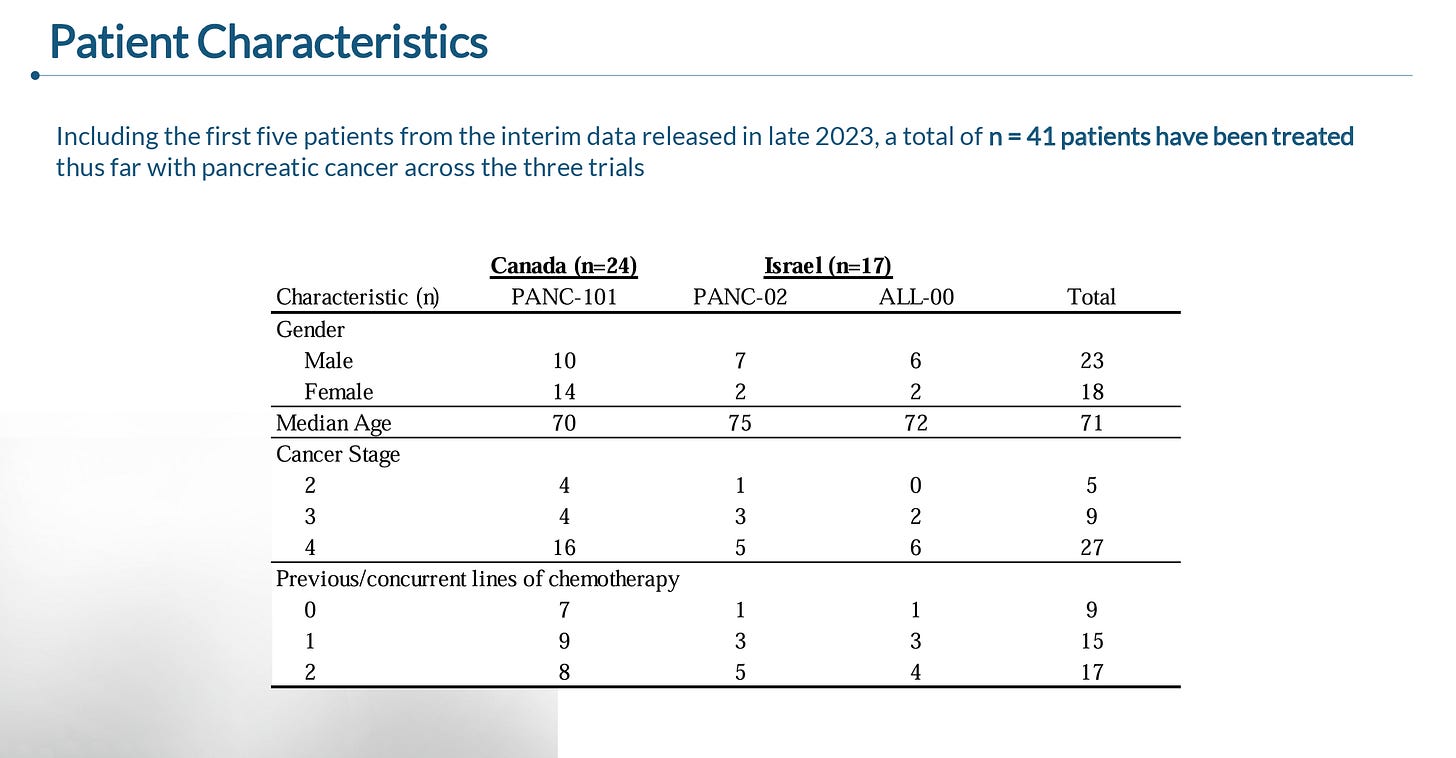
During the January R&D day, the company presented data from 41 patients that were enrolled across three ongoing trials being conducted in Canada and Israel.
As you can see below, the trials included a heterogenous group of patients, though the large majority of patients having metastatic disease and having received at least one line of prior chemotherapy.
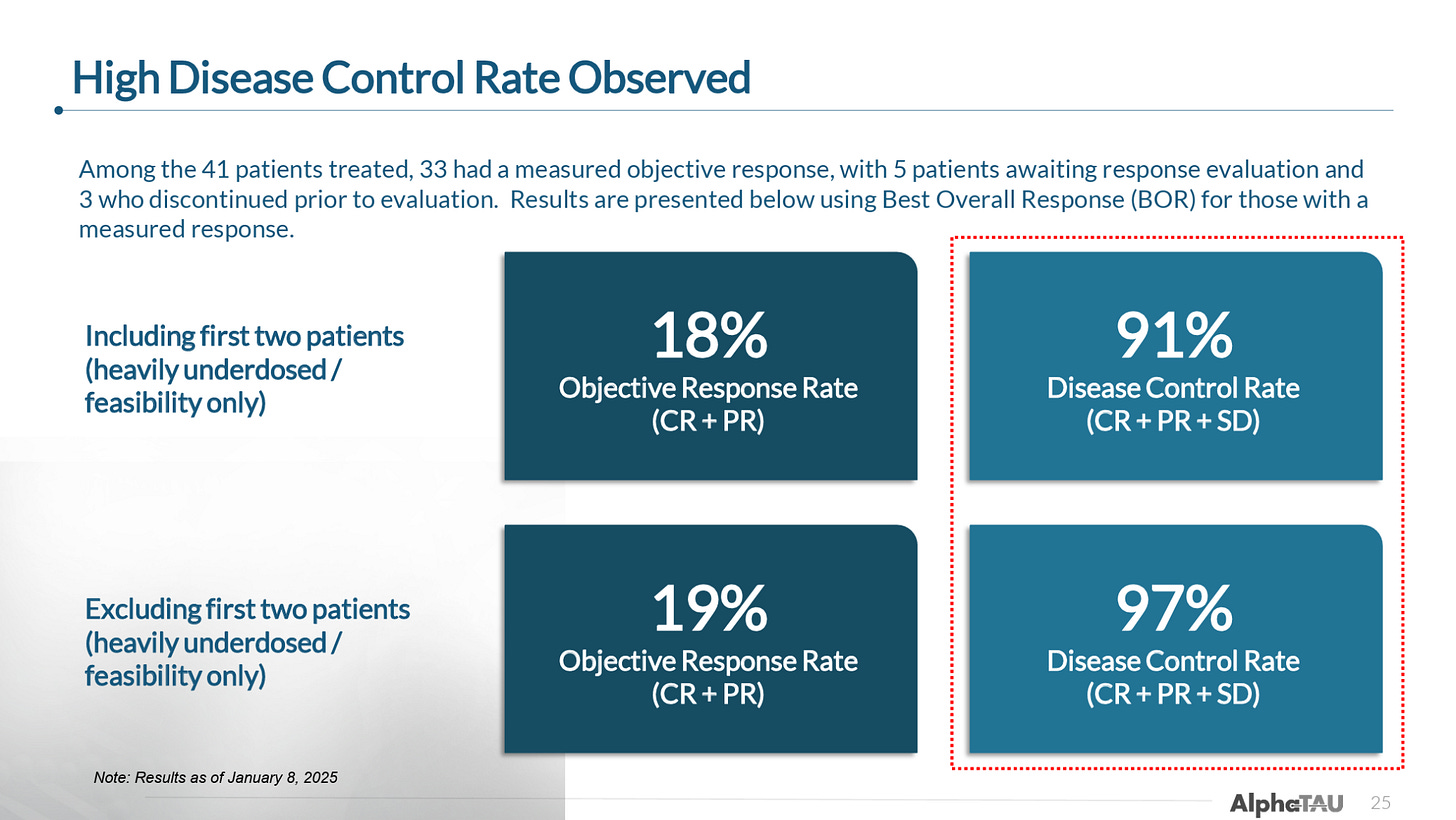
As of the January cutoff, data was available from 33 of the 41 patients (5 had not yet had a follow-up and 3 discontinued the trial), which reported:
91% DCR
18% ORR
When excluding the first two patients treated who received only a very small dose of Alpha DaRT, the efficacy improved to:
97% DCR
19% ORR
So, excluding the two low-dose patients, only 1 out of the 33 patients had progressive disease as their best response per RECIST criteria.
Importantly, Alpha DaRT has also proven to be relatively safe in pancreatic cancer patients, in stark contrast to existing treatment, with zero Grade 4 adverse events, and, notably, zero cases of pancreatitis reported. This speaks to Alpha DaRT’s minimal diffusion into healthy tissue and its potential to be used in combination with other therapies.
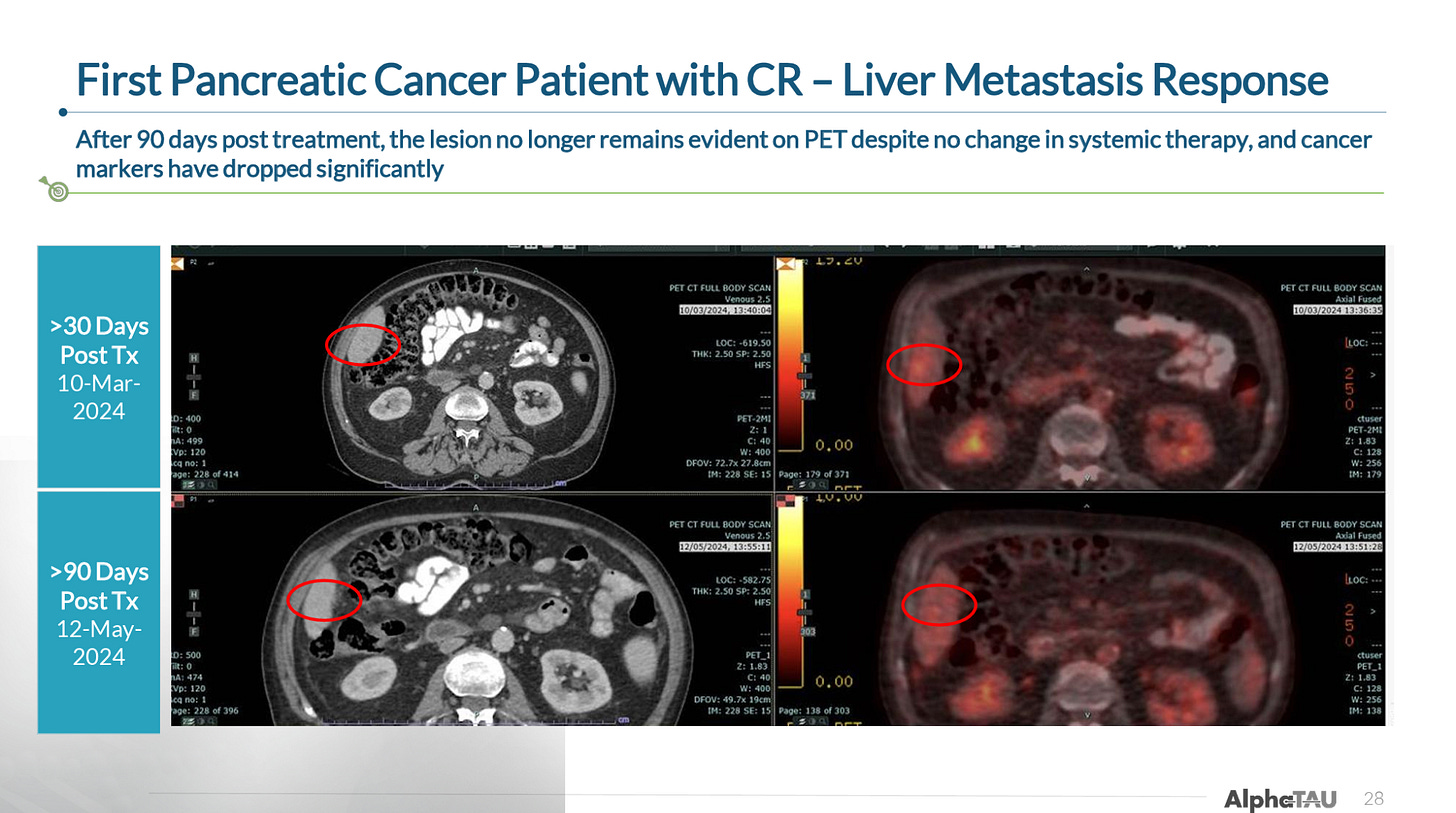
The company also provided a case study from its first CR in pancreatic cancer, in which a patient had both a complete response in the primary pancreatic tumor as well as resolution of liver metastasis.
While these data are already impressive in the context of what is currently available in pancreatic cancer (discussed below), it is important to note that all of these patients were treated with only ~20-55% tumor coverage (the proportion of the primary tumor covered by Alpha DaRT sources) according to the company’s R&D day. The partial coverage was done out of an abundance of caution and as the company and physicians became more comfortable with the use of Alpha DaRT in pancreatic cancer, and suggests that the US trial could report further improved efficacy.
Putting the Data in Context
The company also provided a segmented analysis of the heterogenous patient population to make it easier to put the results into context for different patients groups.
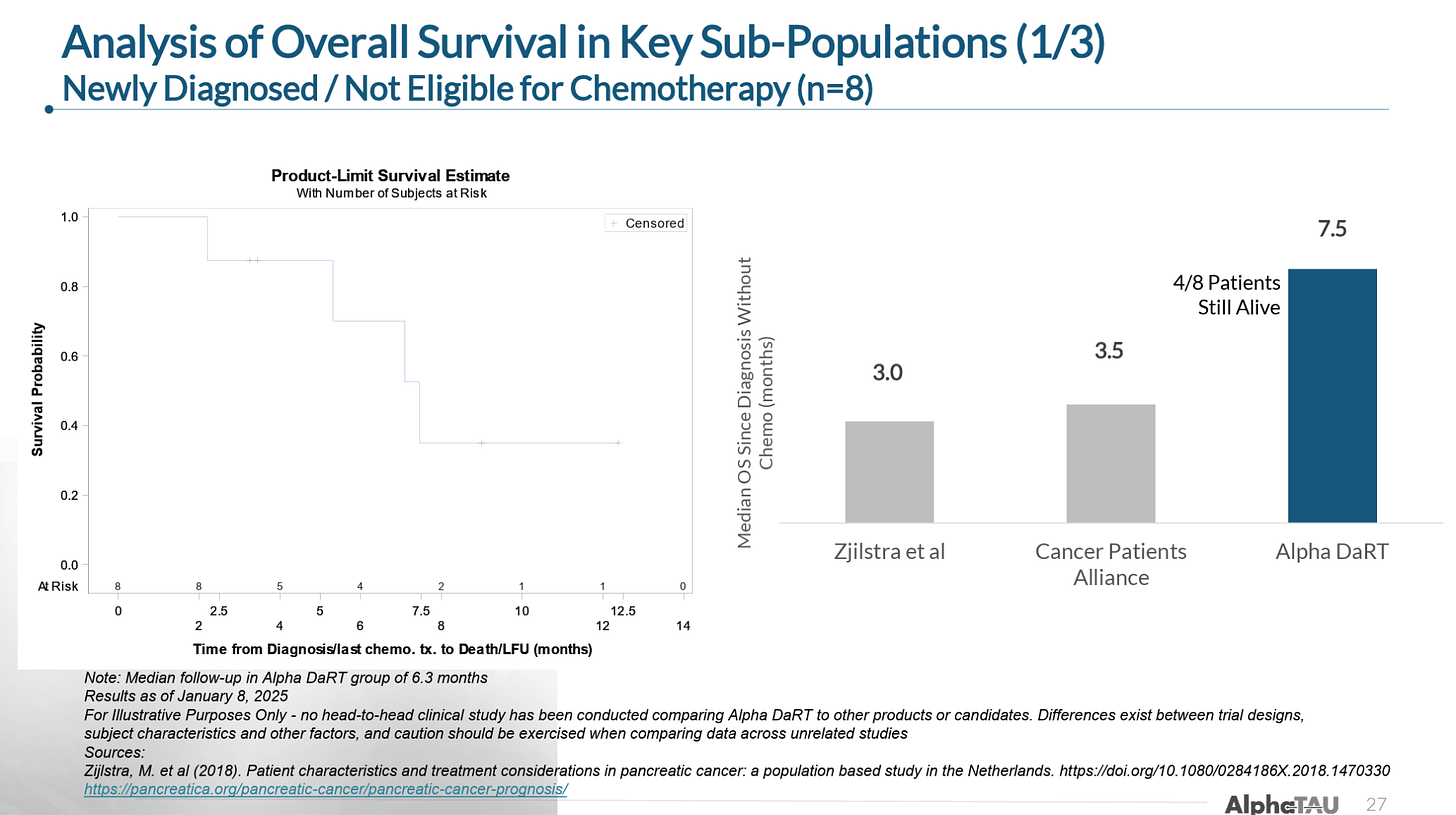
In patients with newly diagnosed pancreatic cancer that were not eligible for (or refusing) chemotherapy, Alpha DaRT delivered 7.5 months median overall survival (mOS) versus 3-3.5 months for standard of care:
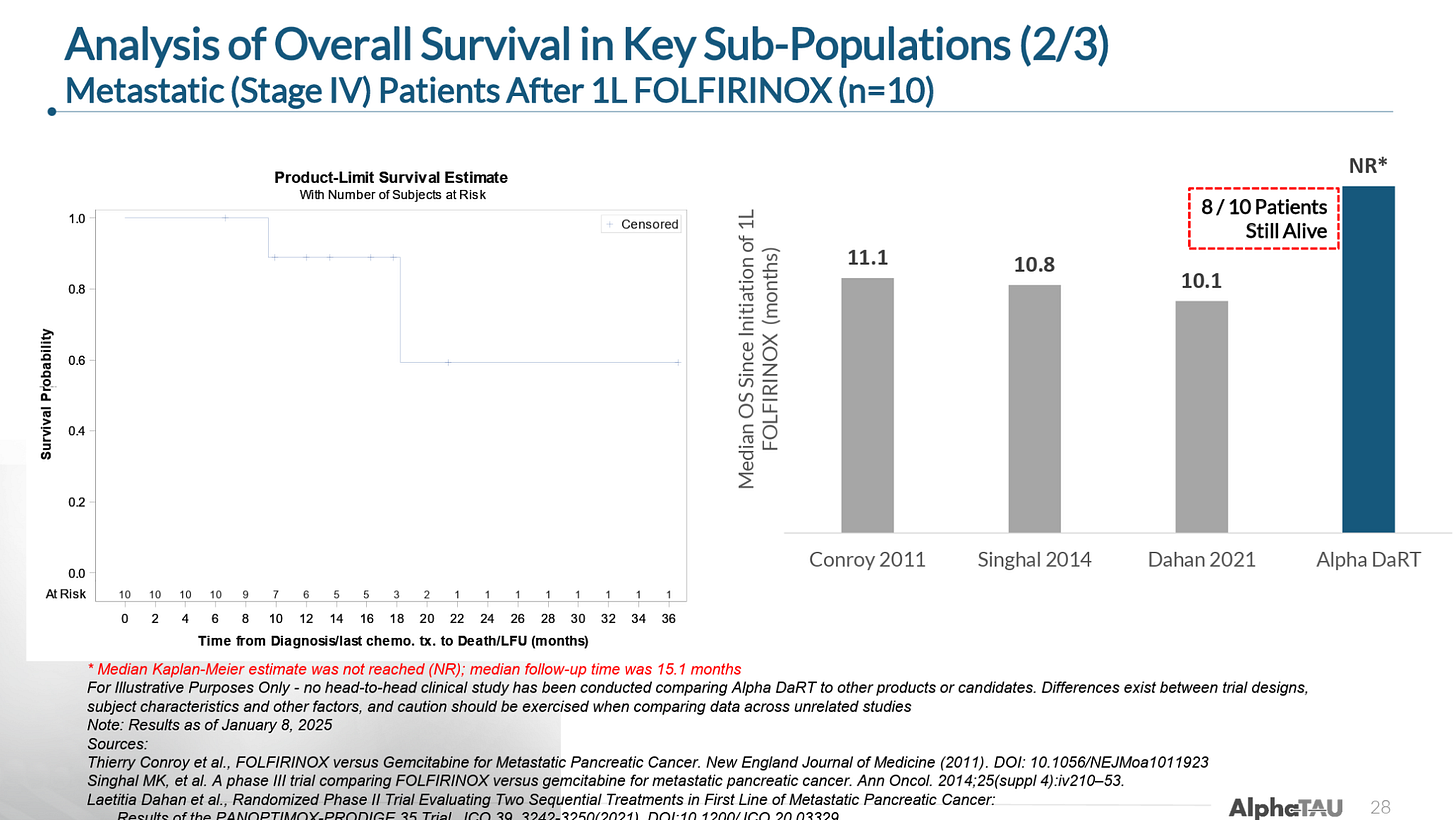
In Stage IV metastatic patients that had received and progressed on first-line FOLFIRINOX (a combination of five chemotherapy agents), Alpha DaRT had extended mOS to at least 15.1 months as of the data cutoff (with 8 of 10 patients were still alive) versus 10-11 months mOS for FOLFIRINOX treatment. (Note: a small number of these patients continued receiving FOLFIRINOX concurrently with Alpha DaRT).
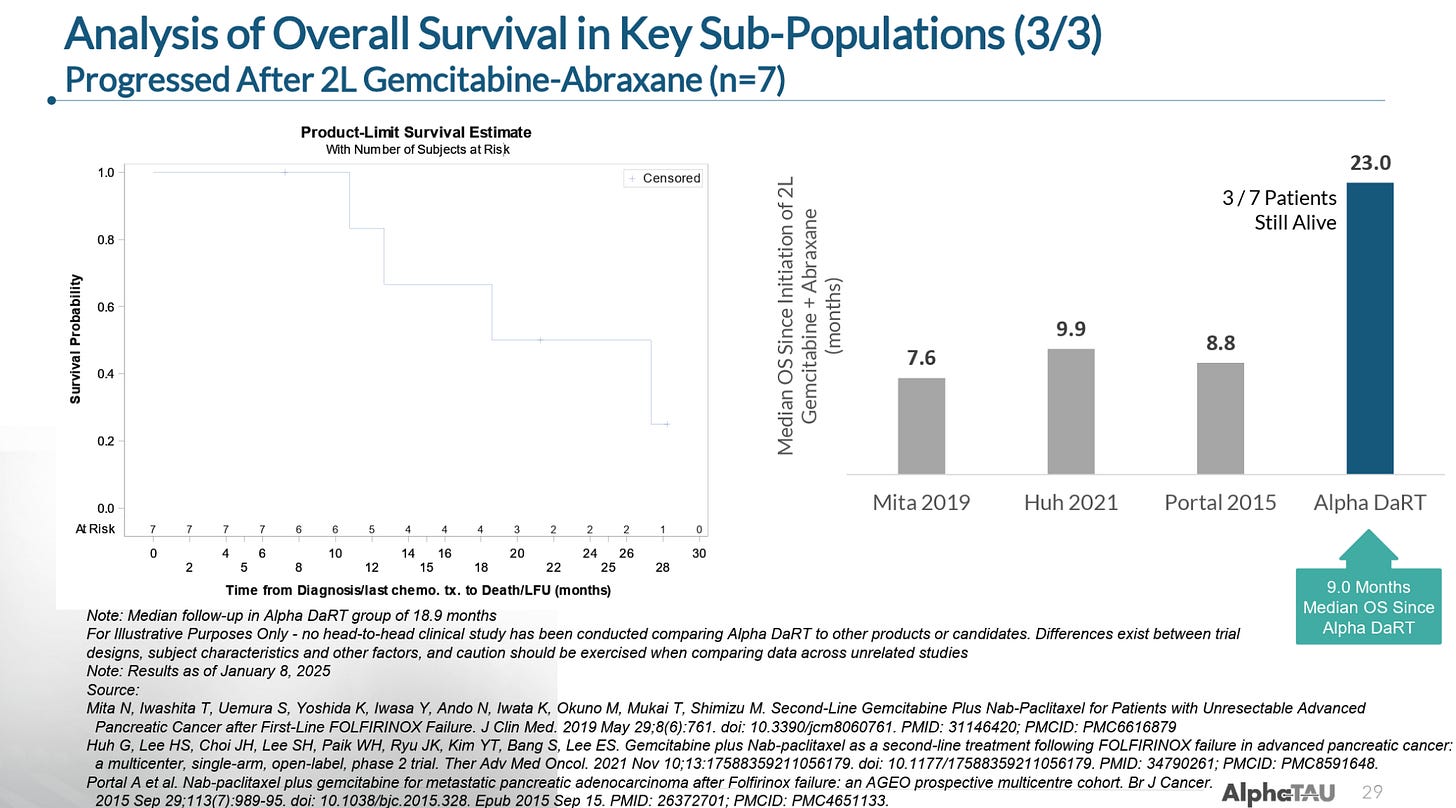
In patients that had failed second-line gemcitabine-piclataxel (GnP), Alpha DaRT extended mOS to 23 months versus the 7.5-10 months of mOS seen with GnP. These patients also had a mOS of 9 months from the time of receiving Alpha DaRT, which means that third-line pancreatic cancer patients with essentially no other treatment options had a 9 month mOS on Alpha DaRT monotherapy.
While the data are a bit messy and difficult to contextualize (e.g. heterogenous patient populations, monotherapy dosing, inconsistent tumor coverage), the early indications suggest Alpha DaRT provides significant improvements over SoC, already more than doubling patient survival in some populations, with room for further improvement as tumors are treated with greater coverage and as Alpha DaRT is trialed in combination with SoC (as in the ongoing US trial).
We expect an update on this pancreatic data in the next few months, potentially in another January R&D day, where we should see longer duration data and some additional patients.
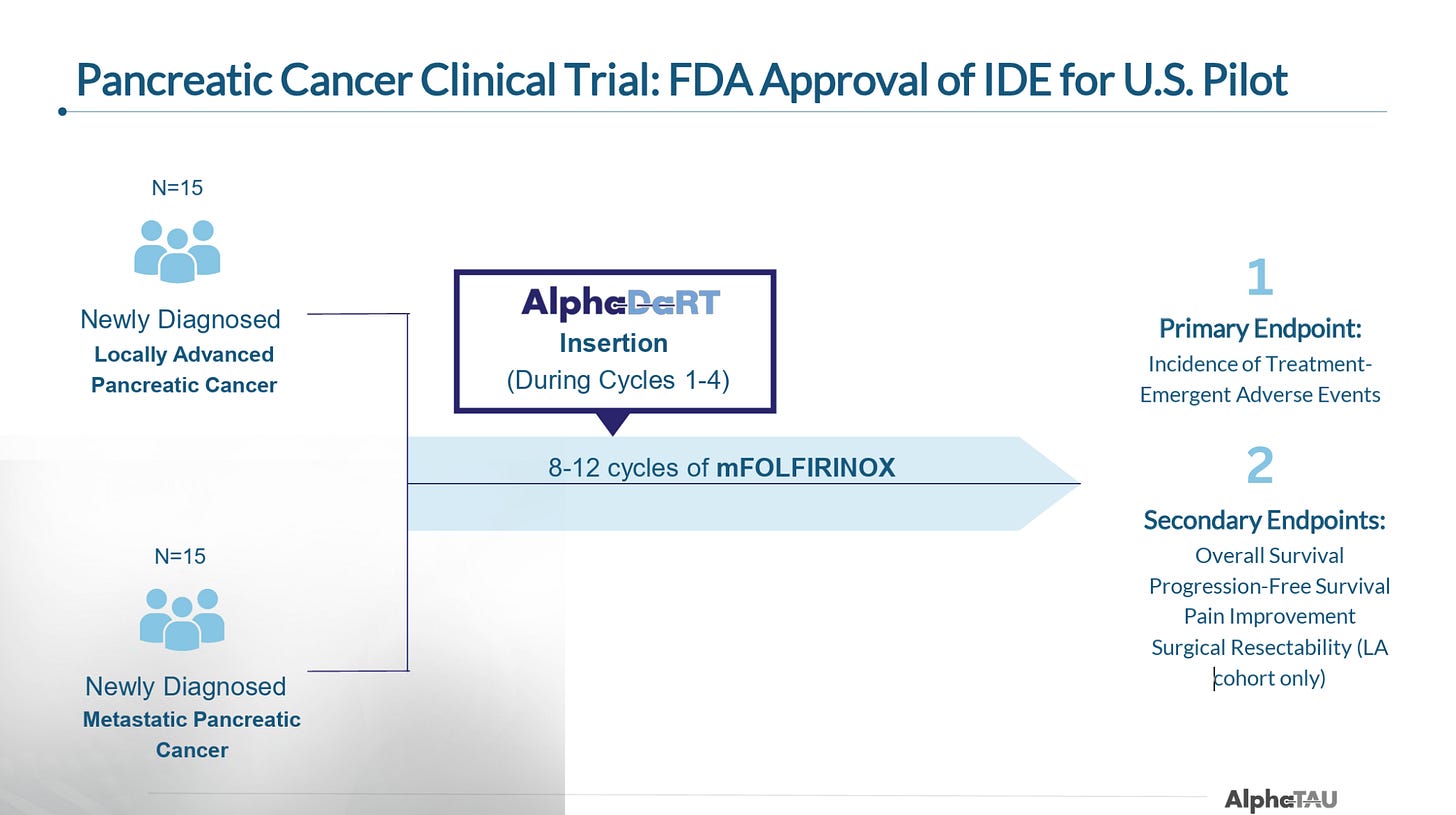
US Trial
In early September, Alpha Tau announced that it treated the first patient in its 30-patient US trial. This trial is set to enroll 15 newly diagnosed metastatic and 15 newly diagnosed locally-advanced pancreatic patients and will administer Alpha DaRT during a course of modified FOLFIRINOX (mFOLFIRINOX). As noted, this will be the first time Alpha DaRT is trialed as in combination with SoC.
The principal investigators on the trial are Dr. Mark D’Andrea and Dr. Isaac Raijman of the University Cancer Center in Houston, Texas, and the trial is enrolling at 16 hospitals, including Emory University, NYU Langone, Montefiore Medical Center.
The company plans to complete enrollment of the trial in the 1Q26, with topline results expected in 3Q25. While patients will likely have to be monitored for longer periods to show mOS superiority vs. standard of care, the company should be able to report response and early PFS (6- or 9-month) data in 3Q26.
Market and Treatment Landscape
There are about 66k newly diagnosed cases of pancreatic cancer each year in the US, up to 87% of which are non-resectable either due to metastatic disease (~50% of newly diagnosed patients) or poor margins.
As was shown above, the two primary treatments for these patients, FOLFIRINOX and gemcitabine + piclataxel (GnP), are fairly very ineffective, extending survival by just a few months. While there are a number of companies pursuing pancreatic cancer there are a few companies with particularly interesting programs we will highlight.
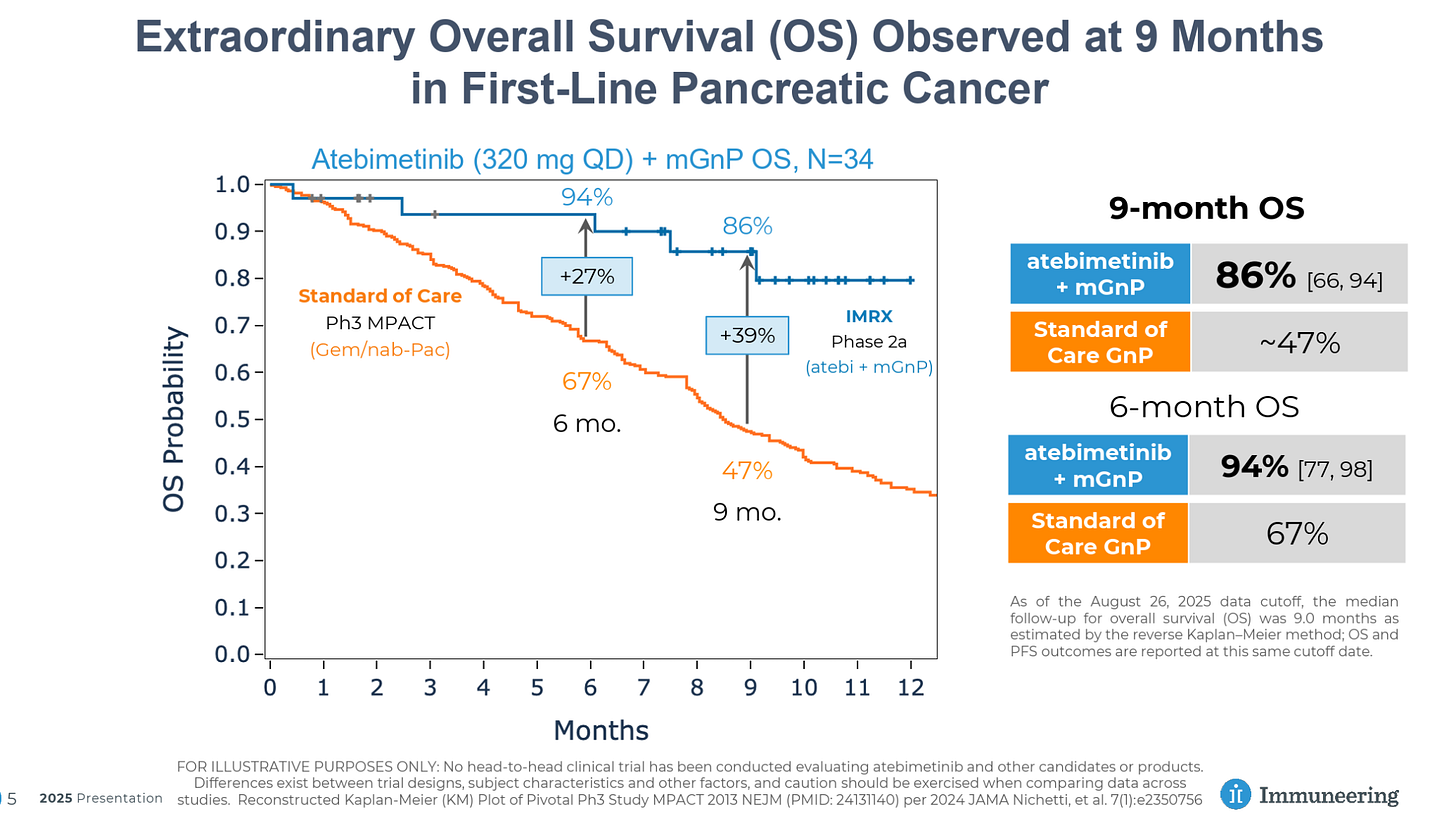
Immuneering
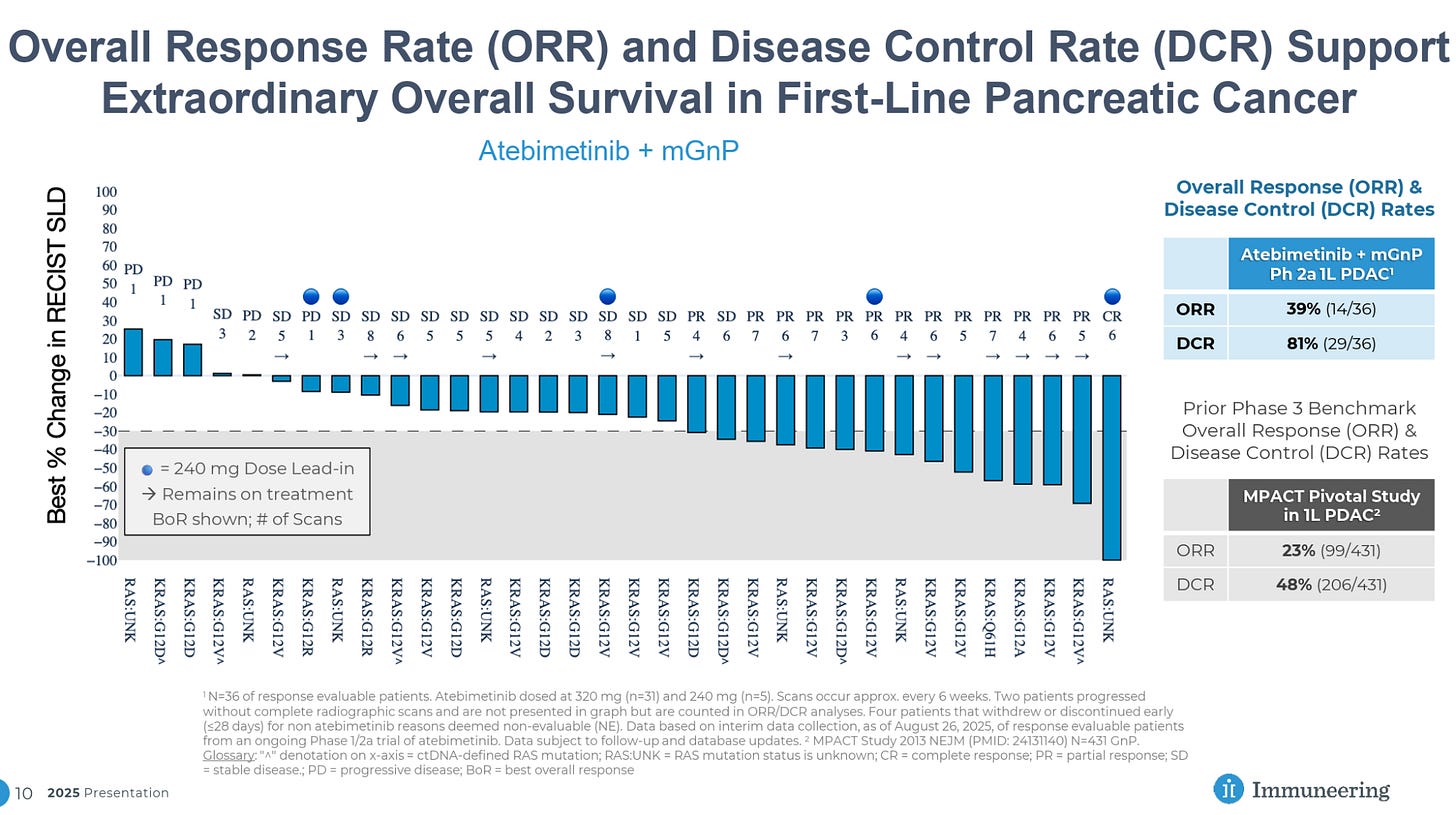
Immuneering’s MEK inhibitor atebimetinib has reported impressive Phase 2a data in combination with GnP in first-line pancreatic cancer patients, showing 86% OS at 9 months versus 47% for GnP alone.
Immuneering also reported impressive response data, including 81% DCR, 39% ORR, and even a CR.
In addition to the CR, Immuneering has also reported that atebimetinib delivered the resolution of a distal bone lesion in a third-line patient in its Phase 1 trial.
Especially for a systemic therapy, these data are quite impressive, with significant improvement versus SoC and a DCR approaching that seen in the Alpha DaRT data to date, though it is important to note that the patient population and treatment settings are not directly comparable.
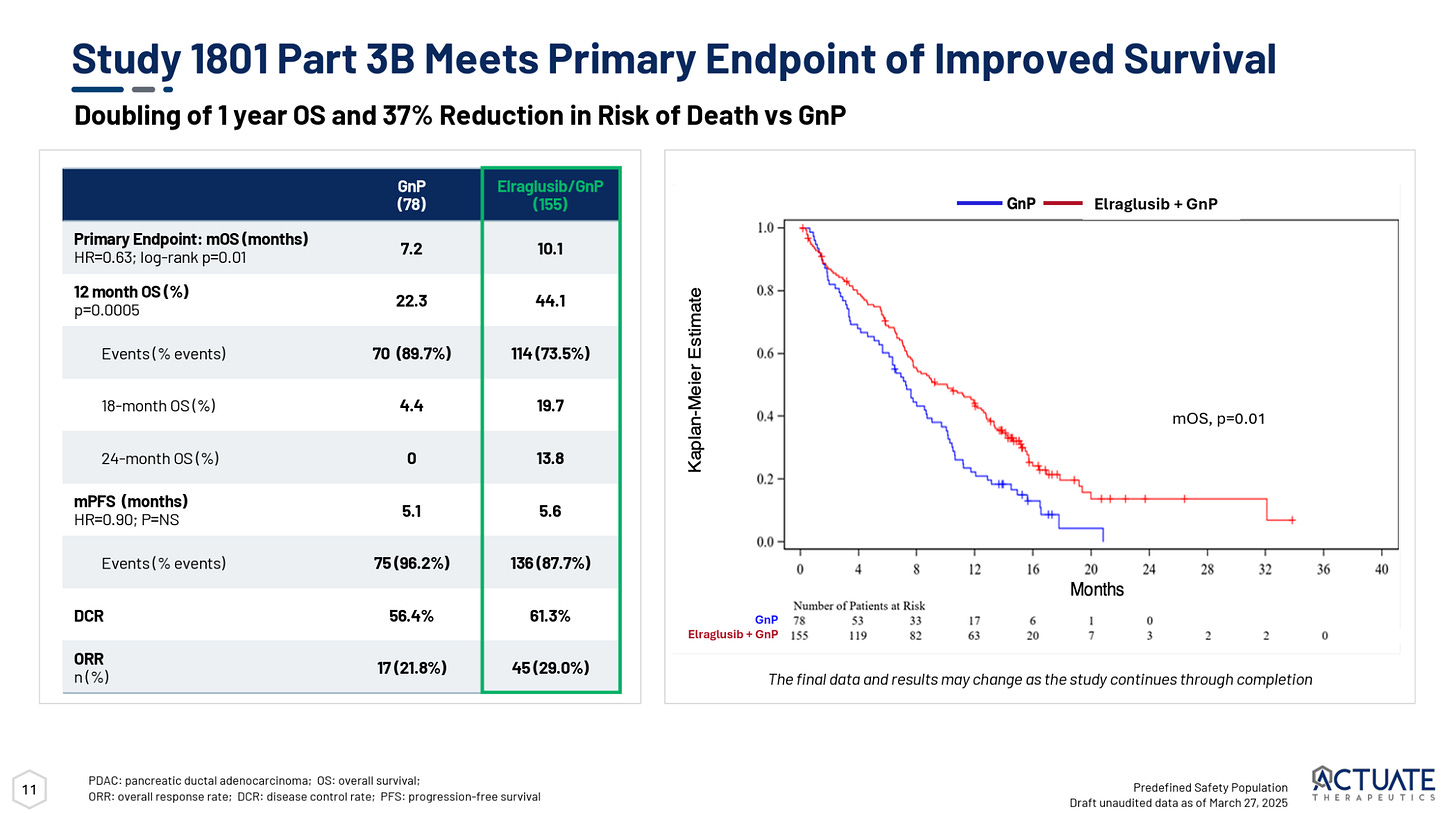
Actuate Therapeutics
In May, Actuate Therapeutics reported Phase 2 data for its GSK-3β inhibitor elraglusib that showed mOS of 10.1 months for elraglusib + GnP versus 7.2 months for GnP in first-line metastatic pancreatic cancer. Additionally, it reported 44% OS at 12 months versus 22% for GnP alone.
While these data represent an improvement over standard of care, they weren’t quite as impressive as Actuate’s earlier Phase 2 trial which showed a 15.3 month mOS and actually reported two CRs.
Both Immuneering and Actuate have reported impressive data in pancreatic cancer and will be interesting to monitor going forward. While the results (particularly from Immuneering) look somewhat comparable to Alpha DaRT’s data in some respects, we would note that the existing data set for Alpha DaRT makes comparisons difficult—Alpha DaRT was being used as a monotherapy in the large majority of patients, was being trialed in later lines of therapy, and as we have said, was using only partial tumor coverage. It will be interesting to see the much cleaner, more interpretable results from Alpha DaRT’s ongoing US trial expected in 3Q26.
Regardless of the fight for superior efficacy, however, Alpha DaRT’s uniqueness as a local treatment with very minimal side effects and potential systemic immune-stimulating effects should make it a key addition to the essentially barren pancreatic cancer treatment landscape, as well as a potentially valuable combination therapy.
Other Indications
Alpha Tau is also set to dose its first patient in a 10-patient proof-of-concept trial in glioblastoma multiforme (GBM). The company developed an intricate delivery device that is able to apply Alpha DaRT directly to tumors in the brain without causing significant damage to brain matter. Alpha DaRT was awarded Breakthrough Device Designation in 2021. The 10-patient study is being led by Ohio State University and is expected to begin enrollment sometime before the end of the year.
Outside of its lead indications, Alpha DaRT is also being trialed in liver, lung, prostate, and vulvar cancer in small pilot studies.
Path Forward and Catalyst Overview
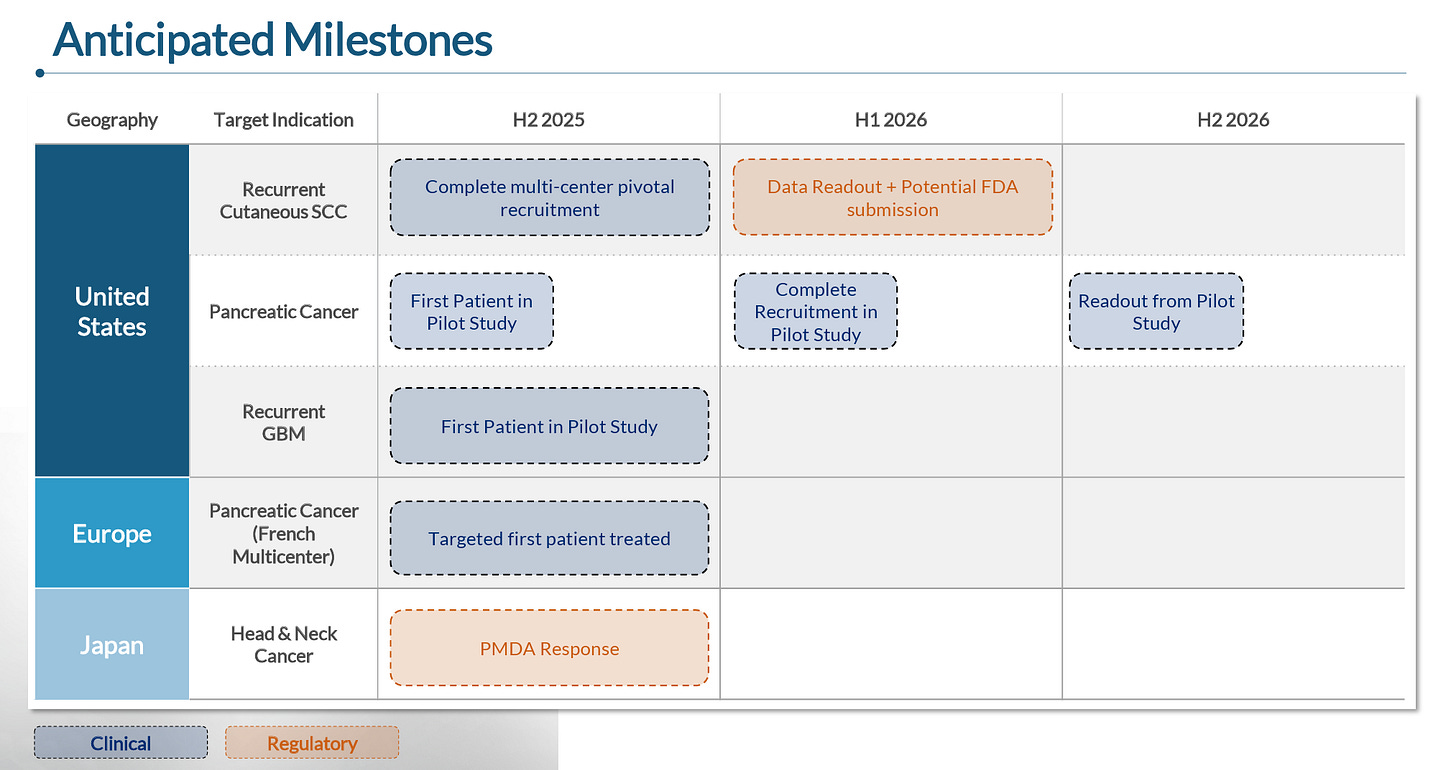
To summarize, Alpha Tau’s should have a rather busy catalyst calendar over the next 12+ months:
Cutaneous SCC
Expects to complete enrollment in potentially pivotal trial around YE25
Data from 86-patient trial expected around mid-2026
May elect to delay commercial launch to prioritize pancreatic and other higher-impact indications
HNSCC
Awaiting determination from Japanese PMDA on approval in HNSCC in Japan; expected 2H25
Potential update on the patients that were enrolled in the pembro combination trial prior to halting (e.g. further durability data and potential additional patients)
Continued consideration on whether to pursue a HNSCC trial in the US, partnership, etc.
Pancreatic
Additional data from ongoing Israeli/Canadian trials (additional patients and durability data) could be presented in coming months
Completion of enrollment in 30-patient US trial in early 1H26, potential topline data in early 2H26
GBM
First patient dosing expected by YE25
Enrollment will be very slow in the beginning, but enrollment could be completed sometime in 2026
Other
Alpha DaRT’s characteristics make it an attractive combination therapy. A partnership, for example in the form of a clinical supply agreement with a checkpoint inhibitor company for a combination therapy trial, seems possible over the next 12 months or so.
In addition to these milestones, we also reiterate that we think Alpha Tau’s general expansion into US-based trials of high-mortality cancers like pancreatic and GBM will increase the company’s profile among both investors and the scientific community.
Financial Position
After the $37 million investment from fellow Israeli biotech Oramed in April 2025, Alpha Tau reported $83 million of cash and equivalents as of 6/30/25. With the company continuing to burn about $6 million per quarter, it should have a long runway, though we note that larger trials in HNSCC, pancreatic, and GBM that may come in the future could increase its burn rate substantially.
As a bit of backstory, Oramed raised over $170 million during 2021 as it advanced a late-stage oral insulin product that ultimately failed. The company currently sits without a real active pipeline, and decided to invest roughly half of its remaining cash ($74 million as of 3/31/25) into Alpha Tau.
Manufacturing
Between its production facilities in Massachusetts and Israel, Alpha Tau currently has the production capacity to cover 1,000-1,500 patients annually. The company is currently building a facility in Hudson, New Hampshire that will be able to supply enough treatment for ~15,000 patients, with the first third of that capacity expected to become operational in early 2026. The company has guided that each of the three phases of the construction will cost in the low single-digit millions.
Comparative Valuation
While Alpha DaRT is completely unique in the oncology landscape as a local treatment that unlocks the benefits of alpha radiation, there are a number of companies that can serve as instructive comps from a potential valuation perspective.
Nanobiotix - $1.3 billion market cap
Nanobiotix, which I covered as a very new analyst back in 2021, is advancing NBTXR3 (now called JNJ-1900), a “radioenhancer” that is injected directly into a tumor designed to enhance the tumor-killing effect of EBRT. In 2023, Nanobiotix licensed NBTXR3 to Janssen (J&J) for up-front and potential milestone payments of $1.8 billion ($2.6 billion once Janssen gained APAC rights from LianBio), as well as low-10s to low-20s tiered royalties.
Nanobiotix has a number of similarities to Alpha Tau:
Utilizes radiation; aims to be tumor-agnostic
Both require patient-specific planning and application from doctors
Potential for systemic immune-sensitizing effects
The drawbacks of Nanobiotix’s technology in relation to Alpha Tau are essentially the same as EBRT:
Toxicity and damage to surrounding tissue limit dosage
Limited to tumors that are accessible with EBRT
Still uses less powerful, less efficient gamma and X-ray radiation
In HNSCC, Nanobiotix recently announced that it delivered a 69% DCR and 34% ORR in combination with a checkpoint inhibitor in recurrent and/or metastatic disease. In esophageal cancer, Nanobiotix recently reported an 85% DCR, 69% ORR, and 46% CR rate in a small 13-patient study. The company also has a potentially pivotal readout in HNSCC expected in the 1H26.
While these results are a welcome improvement on EBRT, they fall significantly short of the data that has been reported by Alpha Tau so far, especially Alpha DaRT’s 80%+ ORR and 30% CR rate in a similar HNSCC population (though a small sample). At the very least, Nanobiotix’s currently $1.3 billion market cap should serve as a benchmark for where Alpha Tau could be valued as it matures, especially considering that the two companies’ timelines for approval are similar. Nanobiotix also serves as an example of an oncology biotech that was ignored for a number of years until investors rushed in as its programs matured, which could be a similar progression for Alpha Tau (especially if it were to engage in a business development of some sort).
Radiopharmaceutical Companies
Radiopharmaceuticals have been a big story in oncology over the past 5-10 years, allowing for targeted delivery of radiation therapy (primarily beta-emitting particles) to tumor cells that express a particular druggable antigen (e.g. PSMA in prostate cancer, SSTR2 in neuroendocrine tumors). Companies operating in the radiopharmaceutical space include Eli Lilly, Novartis, AstraZeneca, BMS, Sanofi, Bayer, many of which entered the space via acquisition in recent years, as well as a number of smaller companies.
While beta particle-emitting radiopharmaceuticals like Novartis’ Pluvicto for prostate cancer ($454 million of 2Q25 sales) and Lutathera for GEP-NETs ($207 million), in recent years there has been a surge of interest in alpha radiation radiopharmaceuticals, resulting in a series of blockbuster acquisitions over just the last two years:
Eli Lilly acquired POINT Biopharma for $1.4 billion in October 2023
BMS acquired RayzeBio for $4.1 billion in December 2023
AstraZeneca acquired Fusion Pharmaceuticals for $2.4 billion in June 2024
Novartis acquired Marian Oncology for $1 billion in May 2024
This flurry of acquisitions took place despite the fact that radiopharmaceuticals are limited only to tumor types for which there is a druggable antigen, which to date has been limited primarily to prostate cancer and GEP-NETs (and some others like lung cancers). As a result, most of these acquired companies are pursuing the same indications.
So big pharma shelled out $8.5 billion of acquisition capital in a ~9 month span for a group of companies that are mostly targeting the same two or three indications, and all of which were in Phase 2 or earlier with their lead assets at the time of acquisition. At the very least, these acquisition price tags should be looked at as very much achievable valuations for Alpha Tau if it can continue to report impressive data.
Merus - $8B market cap
As we discussed previously, Merus was acquired by Genmab for $8 billion last week, primarily because of its HNSCC program, which has Breakthrough Therapy Designation and is conducting Phase 3 trials as both monotherapy and in combination in HNSCC. While Merus is also investigating prostate cancer and some other solid tumors, the acquisition is a testament to how valuable a potential step-change improvement in just one cancer indication can be.
Takeaway
No matter which comparable company you focus on, Alpha Tau’s localized, safe, tumor-agnostic, alpha radiation-enabling technology would appear to deserve far more than a $340 million market cap. We think the stock is simply dramatically underfollowed at current, with average volume at less than 100k shares per day even as it has traded up since the start of August. We expect this Alpha Tau to experience a re-rating as it works through its milestones over the next 12-24 months.
Risks
Delays
Investors that have followed Alpha Tau since its de-SPAC in 2022 will appreciate that some of the clinical development has taken longer than initially expected. The company has been navigating through a new, complex therapy, limited resources, single/few enrollment centers in Israel, the pandemic hangover, etc. that have pushed back some timelines over the years. These delays could continue, especially as US sites begin using Alpha DaRT for the first time.
Unconventional Therapy
Alpha DaRT is an involved procedure, requiring planning both by treating physicians and Alpha Tau, as well as careful application. Although localized therapies are already commonplace in oncology (e.g. EBRT, brachytherapy), the process of using Alpha DaRT might encounter some adoption friction from some doctors that may be more accustomed to using systemic drug therapies.
Pancreatic Data
While Alpha DaRT’s pancreatic cancer data suggests significant advantages over SoC, the complexities and heterogenous nature of the data make it difficult to interpret, especially in the context of other clinical-stage pancreatic companies. On a DCR and ORR basis, the current Alpha DaRT data appears roughly comparable in some ways to some clinical-stage peers, though Alpha DaRT was being trialed as a monotherapy (vs. combo therapy), trialed primarily later line patients, and employed only partial tumor coverage. As a result, there is some uncertainty regarding Alpha DaRT’s true efficacy in pancreatic cancer, which will persist until the US trial reads out in 3Q26. Additionally, while we are optimistic about increased tumor coverage in the US trial, we also note that it is possible that the endoscopic nature of the treatment in pancreatic patients simply makes it difficult to reach full tumor coverage in some patients.
Financial and Dilution
While the company has a decent cash position at ~$80 million, that burn might increase as its US trials kick off. Similarly, the company will likely have to raise additional capital to run larger-scale trials in HNSCC, pancreatic cancer, or GBM in the future.
Investors
The company really doesn’t have any large, well-known healthcare investors. Oramed Pharmaceuticals’ $37 million investment in April of this year was interesting, but, coming from a ~$90 million market cap company, it doesn’t really mean much. We would hope to see the initiation of some large institutional positions in the coming quarters.
The Unknown
Anytime there is a mismatch between a company’s apparent true value and its current valuation in the market, I become fearful that I may be missing some significant flaw in the story. My discussions with management have somewhat satisfied this worry, but there is always the possibility for unknown or unknowable variables to play a role.
Conclusion
While there are a lot of moving pieces to the Alpha Tau story, we think the longer you spend wrapping your head around the data, the more it becomes clear that Alpha DaRT appears to present potentially paradigm-shifting efficacy in oncology, while at the same time being much safer than existing treatment options. While we think the pancreatic data expected around 3Q26 will be a major inflection point that should cause investors to become aware of Alpha Tau, the company has a number of milestones over the next 12 months that should continue to put upward pressure on the stock. At the very least, over the next 12-24 months, we expect Alpha Tau to move from relative obscurity to being acknowledged as a unique and consequential player in the oncology space.


Fantastic write-up, just got time go through it! Thank you so much for your work!