Alpha Cognition: Launching Well-Differentiated Drug into a large Alzheimer's Market Opportunity
**NOTE: Smaller updates and positioning are posted to X/Twitter**
Key Points
Zunveyl nearly eliminates the onerous GI and sleep disturbance side effects of gold-standard AChEI drugs used to treat Alzheimer’s.
With failure of anti-amyloid antibodies and no clear disease-modifying therapies in late-stage development (yet), the $5.5 billion Alzheimer’s market is severely underserved and dissatisfied.
Lean, experienced commercial team launched Zunveyl in March into the long-term care (LTC) market vertical, which accounts for 32% of Alzheimer’s scripts.
Longer term, Zunveyl could become the go-to symptomatic Alzheimer’s treatment, replacing donepzil (Aricept).
In the nearer term, think the company can generate $40-60 million of sales in 2027, which, for a fast growing biopharma company, implies significant upside to the current ~$120 million market cap.
Intro
Alpha Cognition (ACOG) is an relatively unknown pharmaceutical company that just launched its first commercial product, Zunveyl (benzgalantamine), in March for the symptomatic treatment of mild-to-moderate Alzheimer’s disease (AD). After receiving FDA approval in July 2024, Alpha Cognition quietly raised $52 million and uplisted to the Nasdaq in late 2024. Now, still virtually unknown to the investment public (<100k shares average trading volume), the company appears poised to capitalize on a large and underserved ~7 million-patient Alzheimer’s market that has not received a new therapy in 20+ years (excluding mostly-failed antibody therapies).
Zunveyl is a novel formulation of the established acetylcholinesterase inhibitor (AChEI) galantamine, which was originally approved in 2001 as Razadyne under Johnson & Johnson’s ownership. Zunveyl’s novel formulation nearly eliminates the gastrointestinal (GI) side effects that plagued the original galantamine (limiting it to only ~$250 million of peak sales), while also eliminating the insomnia and sleep disturbances frequently associated with current gold standard Alzheimer’s treatment donepezil (brand name Aricept; $2.6 billion of peak sales in 2011). Additionally, there is ample evidence suggesting that Zunveyl’s active moiety, galantamine, is actually the most efficacious of the AChEIs due to its unique dual mechanism of action. As a result, we believe Zunveyl has the potential to unlock the efficacy benefits of galantamine and become the clear best-in-class symptomatic treatment for Alzheimer’s over time.
Alpha Cognition’s lean but impressive 32-rep sales is initially launching into the long-term care vertical (LTC; nursing homes, memory care facilities, etc.), which the company has selected as its initial target market due to its size (~800k patients) and prescription density (accounts for 36% of the 11 million prescriptions annually). Management has made multiple encouraging comments about early interest from physicians, including inbound interest from prescribers and positive feedback about the impressive clinical profile of Zunveyl. There will likely be further updates on the 1Q25 earnings call after the close on Thursday May 15th.
While real revenues likely won’t begin to register until the latter half of 2025 and into 2026, CEO Michael McFadden has commented on his expectation of a potential “hockey stick” revenue acceleration once physicians get educated on and comfortable with Zunveyl. Importantly, Alpha Cognition believes its ~$55 million of cash (as of 12/31, pro forma for CMS Pharma out-licensing) is sufficient to reach profitability, which it expects to achieve in year three of Zunveyl’s launch (~2027). Given what we perceive as management’s tendency to speak conservatively, this guidance not only implies a strong revenue ramp, but would also insulate the company from the financing overhang that plagues many small/micro-cap biopharma companies.
While the majority of interest in the Alzheimer’s space is shifting from mostly-failed antibody therapies to inflammation-related disease-modifying therapies (which we believe will eventually succeed and probably pose a medium-term risk to Zunveyl’s total addressable market), the $5+ billion symptomatic Alzheimer’s treatment is sorely in need of a potential step-change solution like Zunveyl. We also note that Zunveyl will have essentially no competition from branded products during its launch.
Alpha Cognition presents a unique opportunity as a clinically de-risked, financially-insulated early commercial-stage biopharma company launching into a massive yet significantly underserved Alzheimer’s market with a drug in Zunveyl that we believe has the clinical profile to potentially replace donepezil/Aricept as the gold standard symptomatic treatment for Alzheimer’s disease over the long term.
In the near term, we believe Alpha Cognition can deliver $40-60 million of sales in 2027, which, given we expect continued growth and rapidly scaling profitability from 2027 onward, could fetch anywhere from a 5x to 10x sales multiple. This would imply roughly 100%-400% upside versus Alpha Cognition’s current market cap of ~$120 million and represents what we believe to be a significantly asymmetric risk-reward opportunity.
History of AChEIs
It is useful to give an overview of AChEIs, which revolutionized the treatment of Alzheimer’s patients with their introduction in the mid-1990s, before which there was essentially no pharmacological treatment available for the management of Alzheimer’s disease. While AChEIs are not curative (i.e. they are symptomatic treatments), they provide something like 1-2 years’ worth of cognitive improvement versus baseline and/or cognitive stabilization, which can allow greater independence, improve interactions with loved ones, etc.
Today, AChEIs, including their combination with 2003-approved NMDA receptor antagonist Namenda (memantine), still account for 90%+ of Alzheimer’s prescriptions, with donepezil/Aricept being by far the most popular (estimated 65% market share). With what we regard as the failure of anti-amyloid antibody therapies like Aduhelm (withdrawn from the market by Biogen), Lequembi, and Kisunla that were meant to be disease-modifying, amazingly, there has been essentially no change in the treatment/management of Alzheimer’s since 2003.
History of Alzheimer’s Disease Management Drugs
As you can see, the winners in the Alzheimer’s space have done quite well from a commercial perspective, with Aricept generating $3.5 billion in peak sale primarily due to its tolerability, which, despite being far from ideal (discussed in next section), is superior to other AChEIs.
You can also see that even less differentiated drugs like the Exelon patch (transdermal formulation of rivastigmine) and Namzaric (a simple combination of donepezil and memantine) were able to achieve $625 million and $267 million of peak sales, respectively, demonstrating just how large the Alzheimer’s opportunity is.
The Problem: Side Effects
Despite commercial success, AChEIs—due to their mechanism of action on the cholinergic system, which controls things like digestion, alertness, and memory—have been plagued by tolerability issues. Even donepezil, the most tolerable of all of the AChEIs, presents major side effects in some patients.
The following side effect rates are taken from the respective product labels:
Some studies and doctor reports also indicate that the observed side effect rates of AChEIs can be higher in practice. Specifically for donepezil/Aricept, while the label reports a 9% rate of insomnia, this study reports that 25% of patients taking donepezil in the morning experienced night time disturbances (NTD; e.g. insomnia, nightmares, etc.), while 48% of patients taking donepezil at night experienced insomnia, nightmares, or other sleep disturbances.
In addition to being unpleasant for patients, insomnia and other NTDs lead to a number of second-order consequences. For instance, lack of quality sleep in Alzheimer’s patients can hasten the deterioration of the condition (link), as well as put patients at risk of wandering at night, psychological symptoms, and increased fall risk.
On the other hand, GI side effects lead to poor appetite and difficulty digesting food, which makes it more difficult for patients to maintain their weight and strength, increasing their chance of falling, which can be catastrophic in fragile patients.
Both night time disturbances and GI side effects also significantly increase the burden on caretakers (both paid and unpaid) who have to help patients go to the bathroom, clean up messes, watch for night time wandering, etc.
Zunveyl
As a novel formulation of galantamine, Zunveyl (benzgalantamine) was approved via the 505(b)(2) pathway based on four bioequivalence and bioavailability studies which showed pharmacokinetic equivalence with galantamine.
Zunveyl’s formulation differs from the original galantamine formulation in two key ways:
Prodrug formulation: The addition of benzoic acid to the molecule allows Zunveyl to remain inactive until it is absorbed into the bloodstream and is processed by the liver, avoiding intestinal irritation.
Enteric coating: Coating around the prodrug that protects it from degradation by stomach acid and allows for delayed release.
This formulation led to Zunveyl’s four bioequivalence studies (in 150 healthy volunteers) demonstrating a significantly favorable side effect profile versus galantamine. On GI side effects, the company reported just 2% incidence of diarrhea and 1% incidence of nausea and vomiting:
On sleep disturbances, Zunveyl reported no incidence of insomnia across the 150 healthy volunteers tested. While galantamine was already pretty benign with regards to insomnia, this compares very favorably to the 10-40% incidence of sleep disturbances reported for donepezil across various studies. Those interested in the details of Zunveyl’s studies can find a more detailed explanation on pages 6-8 of the company’s 2024 10-K.
With 1-2% rates of GI side effects and no incidences of insomnia (caveated by the fact that these results came in healthy volunteers), Zunveyl appears to nearly solve the side effect problem that causes significant symptoms for patients and dissatisfaction in the Alzheimer’s care market.
Efficacy and Galantamine Data
From an efficacy and mechanistic perspective, Zunveyl is also supported by some very interesting data on galantamine which could support broad adoption longer-term. There are three key areas:
1. Galantamine is the only AChEI with a demonstrated dual mechanism of action.
In addition to being an acetylcholinesterase inhibitor (AChEI), which simply inhibits the breakdown of acetylcholine, increasing its availability for receptor binding, galantamine is also a positive allosteric modulator (PAM) of α7 and α4β2 nicotinic cholinergic receptors. These receptors are specifically involved in memory, alertness, and cognition (link).
The other interesting aspect about galantamine’s mechanism is that it actually has relatively weak acetylcholinesterase inhibition compared to other AChEIs, with studies finding 3-15x weaker inhibition for galantamine vs. donepezil (link).
This, combined with the allosteric modulation of α7 and α4β2 receptors, essentially means that galantamine is heavily biased towards specific stimulation of α7 and α4β2 receptors (rather than equal stimulation across all acetylcholine receptor subtypes). This likely lends galantamine its unique and apparently superior efficacy profile versus other AChEIs (discussed next), as both the α7 and the α4β2 receptor have been shown to be critical for synaptic plasticity, learning, and memory:
Galantamine’s selective mechanism may also underpin its sleep-promoting effects (discussed below). While AChEIs like donepezil which increase stimulation of all acetylcholine receptors which may lead to overactivation and reduced non-REM deep sleep, galantamine’s bias towards α7 and α4β2 nicotinic receptors may increase sleep-promoting chemicals like GABA and serotonin in the brain (link, link, link) .
2. Galantamine has proven to preserve/benefit cognition more than other AChEIs.
One of the key papers that Alpha Cognition cites as a differentiator for Zunveyl is this paper by Dr. Hong Xu from the Karolinska Institute in Sweden: Long-term Effects of Cholinesterase Inhibitors on Cognitive Decline and Mortality (2021)
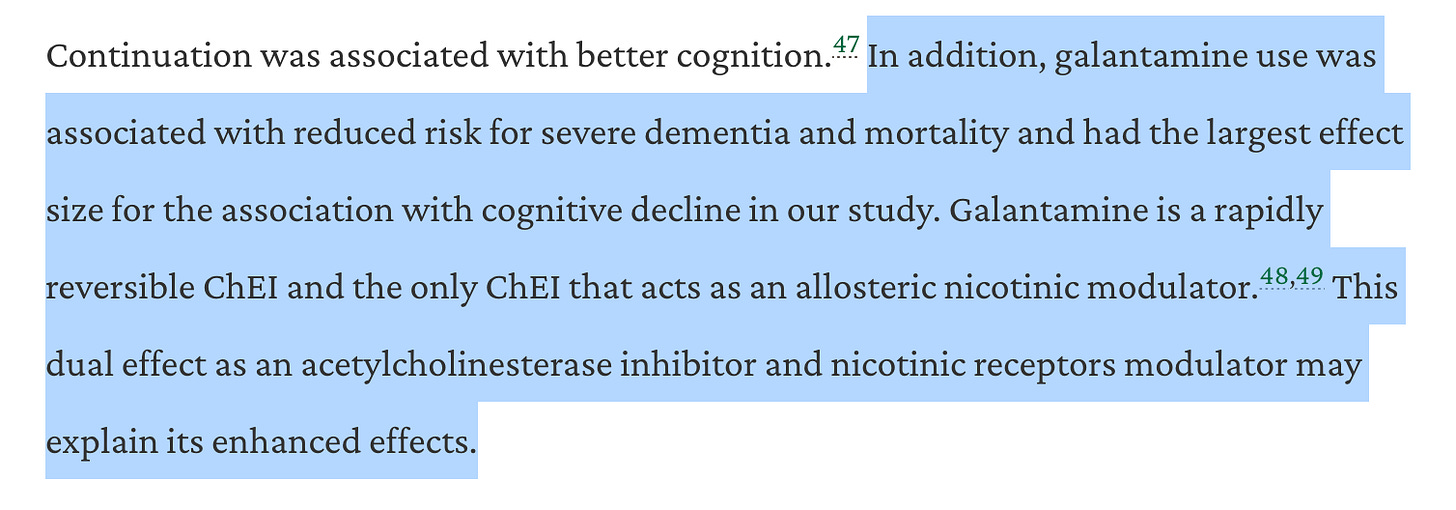
The paper, which was published in Neurology, analyzed 39k Alzheimer’s patients. Investigating long-term outcomes with patients on donepezil, rivastigmine, and galantamine, the study found that “Galantamine was the only ChEI that demonstrated a significant reduction in the risk of developing severe dementia, in addition to presenting the strongest effect on cognition.”
The article clarifies:
The authors also noted that efficacy was stronger at higher doses, meaning a more tolerable formulation of galantamine could unlock further efficacy advantages from galantamine.
Other studies supporting galantamine’s efficacy advantage:
Comparison of cholinesterase inhibitor safety in real-world practice (2019): “Galantamine users experienced longer independent living.”
Galantamine: Effect on nicotinic receptor binding, acetylcholinesterase inhibition, and learning (2001): “Among the many cognition-enhancing drugs we have tested in 4-month-old rabbits (BMY-21502, donepezil, GTS-21, nefiracetam, nimodipine), Gal is the only drug that has facilitated learning in young rabbits.”
3. Galantamine has been shown to be the most pro-sleep AChEI.
The evidence seems to clearly support the notion that galantamine improves sleep in Alzheimer’s patients both versus baseline, but especially in patients that are taking donepezil.
Conclusion
This data, which only scratches the surface, supports the idea that galantamine could have been a best-in-class Alzheimer’s drug if not for its high GI side effects, and that Zunveyl could represent a realization of this potential. The data also arms the Alpha Cognition’s sales team with ample evidence with which they can demonstrate an attractive clinical profile to HCPs.
Zunveyl: The Next Aricept?
In a longer-term upside scenario we believe Zunveyl could start to replace donepezil as the go-to Alzheimer’s drug due to its superior clinical profile. There are even studies directly finding galantamine can be more efficacious than donepezil:
A long-term comparison of galantamine and donepezil in the treatment of Alzheimer's disease (2003): “Significant advantages were found in the treatment response to galantamine (versus donepezil) on cognition as measured by response rates on the MMSE and ADAS-cog11.”
Predicting the neural effect of switching from donepezil to galantamine based on single-photon emission computed tomography findings in patients with Alzheimer's disease (2016): This study shows that patients switching from donepezil to galantamine showed reduced apathy, improved executive function, and improved motor skill.
Galantamine-memantine combination superior to donepezil-memantine combination in Alzheimer’s disease: critical dissection with an emphasis on kynurenic acid and mismatch negativity (2018): “In a retrospective study in AD, the galantamine-memantine combination significantly improved cognition compared to the donepezil-memantine combination.”
Given these results, some may wonder why donepezil completely dominated the AChEI market. The explanation, which is supported by this 2004 study, is that galantamine’s side effects and the associated challenges were simply too burdensome.
While supplanting donepezil as the go-to Alzheimer’s treatment will take years, pairing all of galantamine’s efficacy data with Zunveyl’s improved tolerability suggests it would improve outcomes for patients, caretakers, and loved ones.
Alzheimer’s Market Opportunity
There are ~7 million Alzheimer’s patients in the US, with ~11 million prescriptions written annually. At a wholesale acquisition cost (WAC) of medication ~$500 per month, this represents an estimated ~$5.5 billion annual market in Alzheimer’s disease treatment.
Within the Alzheimer’s market, the company is initially launching in the LTC market (e.g. memory care units, skilled nursing facilities, and other residential care homes), which it believes account for ~13% of the AD population (~800k patients), but contribute 36% of all Alzheimer’s prescriptions.
The company arrives at an estimated addressable market of $2 billion by multiplying the 36% share of prescriptions coming from LTC by the 11 million total annual prescriptions by a WAC for existing treatments of $500. Given that Zunveyl’s price is set at $749 per month, the actual total addressable market in LTC may be closer to $3 billion.
The size of this opportunity is only going to increase as the population of people aged 65+ with Alzheimer’s in the US is expected to nearly double from 6.1 million in 2020 to 11.2 million in 2040 (link).
Patient and Caregiver Dissatisfaction
The side effects of AChEIs create significant dissatisfaction among both patients and caregivers that present a large opportunity.
Alpha Cognition believes based on market research that ~55% of patients discontinue their AD medication due to side effects. This lines up with this study which found a 53% discontinuation rate for donepezil specifically. Other studies have found lower rates like 21%, though the observation period was shorter (one year).
According to the company’s market research 72% of doctors are dissatisfied with current treatment options, primarily due to treatment side effects. As a result, 88% of LTC HCPs said they would be likely to prescribe Zunveyl in the company’s market research.
LTC Market Challenges
LTC facilities can be some of the most grueling verticals in healthcare due to the inherently difficult nature of patients’ conditions, and the situation has only worsened since COVID.
As a result of difficult conditions exacerbated by COVID-19, nurses, CNAs, and other healthcare workers have fled the LTC setting in record numbers, leading to labor shortages.
As a result of understaffing, a study showed that 26% of patients in LTC facilities in Canada are given antipsychotic medications (link), which are not approved for treating Alzheimer’s or dementia and actually carry a black box warning for their ability to increase stroke risk in patients with dementia.
Zunveyl, if it can improve behavior and lower challenging side effects for patients, could be a godsend for these healthcare workers.
Insurance Coverage
The company has shared that 65-70% of LTC patients already have access to Zunveyl through their health insurance with zero copay.
Discussions with payers during the 1Q25 also led to the company selecting $749/month as the monthly WAC for Zunveyl, above what it had previously expected, indicating acknowledgement of the need for new treatment options by health insurers.
The company reports that hurdles for prescribers are minimal and expected for a new drug, with most insurers requiring a prior authorization as the main hurdle. While the prior authorization creates some friction for prescribers, the company aims to streamline the process, and will look to negotiate with insurers to eliminate it starting in 2026.
Additional Market Verticals
After LTC, Alpha Cognition plans to expand its commercialization efforts to the Neurology setting (e.g. neurologists, psychiatrists), which represents another 27% of annual prescriptions. Together, LTC and Neurology represent almost two-thirds of prescriptions for Alzheimer’s.
Unmedicated Patients
As we covered, the vast majority of Alzheimer’s patients taking medication are treated with AChEIs (80%+), primarily because there are no alternatives other than memantine (which is not commonly used as a monotherapy).
But it is important to note that, as a result of the side effects of AChEIs, a surprising proportion of patients are unmedicated—estimates vary, but 11 million annual prescriptions implies only 916k full-year patients on medication, which is only about 13% of the 7 million Alzheimer’s patients in the US. Patients going unmedicated not only potentially decreases their functionality and independence, but patients treated with AChEIs have been shown to experience slower cognitive decline and lower rates of mortality than unmedicated patients (link).
The improved safety profile of Zunveyl may allow unmedicated patients to benefit from the positive effects of AChEIs.
Caregivers
Nearly 11 million Americans provide unpaid care for patients with Alzheimer’s or other dementias. These amount to an estimated 18.4 billion hours of informal, unpaid work every year, representing $347 billion of value (link).
In addition to the burden on understaffed LTC healthcare workers, the burden on these unpaid family members, spouses, and friends is substantial. For example, 59% of family caregivers to Alzheimer’s or other dementia patients rated the emotional stress of caregiving as high or very high.
Some other stats regarding caregiver burden:
Conclusion
In all, the Alzheimer’s market is large and well-established, but significantly underserved, leading to an extreme quality of life burden for patients, healthcare workers, and caregivers. The density of patients and the clear dissatisfaction of HCPs in the LTC facility vertical make it an especially attractive market for a product like Zunveyl, and one that can be addressed by a relatively small team.
Commercial Team
Collectively, Alpha Cognition’s sales team, which is relatively lean at just 32 representatives, brings a combined 330 years of commercial experience, 150 years of experience specifically in the LTC vertical, and 140 product launches. Collectively, the team has an average of 16 years of biopharma industry sales experience and 10 years specifically in LTC. You can learn more about the individual salespeople here.
Despite a relatively lean team, Alpha Cognition believes it can initially address 80%+ of the LTC segment by focusing on key geographies.
The commercial team is led by Chief Operating and Commercial Officer Lauren D’Angelo, who has a lengthy track record on the commercial side of the biopharma industry, including 10 years at AstraZeneca where she rose from a sales rep to Brand Director of CNS, Pain, and GI. Lauren comes across as very motivated, proactive, and experienced in the company’s presentations.
Other members of Alpha Cognition’s commercial team that bring 20+ years of commercial biopharma experience are Jennifer Pesa (Eli Lilly, Abbott, Otsuka, Sumitomo), Greg Anderson (Intra-Cellular, Urovant, Otsuka), Trierre Shanks (AstraZeneca, Sun Pharma, Sumitomo, Bristol Myers), Michael Spence (Sanofi, Novartis, Avanir, Sumitomo), Jennifer Lane-Watson (Eli Lilly, Avanir, Urovant, Sumitomo), and Amy Kincaid (Avanir, Urovant, Ionis). As you can see, many of these executives have prior and overlapping experience working together.
In March, Alpha Cognition held a kickoff sales meeting and launched Zunveyl, and you can really feel an energy and excitement from the team across all of the posts and comments on LinkedIn (link, link).
Perhaps the team is excited because they know that Zunveyl’s clinical profile and the highly underserved market are a unique opportunity (for salespeople and investors alike).
Launch
Early comments from management regarding the launch appear encouraging. For starters, the late-stage pricing discussions with insurance companies in 1Q25 led to the company setting a $749/month WAC, above the company’s original expectation of $500-$650/month, suggesting health insurers recognize the unmet need that Zunveyl is addressing.
Lauren D’Angelo also commented that initial interest has been strong.
She also commented that doctors seem quite interested in Zunveyl, more than for products she has worked with in the past, and that the doctors are “literally saying ‘you’re going to sell a ton of this’”.
The CEO has also commented that he expects a “hockey stick” uptick in adoption, presumably in 2026 and 2027.
With Zunveyl’s unique characteristics and these early indications of interest from physicians, we think there could be a sort of “network effect” that takes hold amongst physicians in the LTC space sometime in 2026 once enough physicians have been educated and tried the product on their patients.
We will likely learn more about early adoption on the May 15th call.
Alzheimer’s Market Comps
There are some key historical examples of new entrants to the Alzheimer’s space that can be instructive in thinking about Zunveyl’s adoption curve. While blockbusters like donepezil/Aricept and memantine reached $1+ billion blockbuster status as best-in-class drugs, products with much less differentiation like the Exelon Patch and Namzaric were still able to reach $650 million and $270 million of peak annual sales, respectively.
Exelon Patch
The Exelon Patch, introduced in 2007, was a transdermal formulation of rivastigmine (Exelon) meant to limit the GI side effects.
While the low dose of the Exelon Patch lowered the incidence of nausea versus oral rivastigmine from about 23% to about 7%, at higher doses, it caused GI disturbances that were basically in line with rivastigmine itself, reporting 21% rate of nausea and 19% rate of vomiting (worse than the oral drug). You will also notice that diarrhea rates were worse with the patch, even at the lower dose (link).
With a modestly improved side effect profile over rivastigmine (which itself is usually a second choice after donepezil), the Exelon Patch was able to nearly triple oral Exelon’s peak sales, reaching over $600 million of annual sales in 2014 (before losing market exclusivity in 2015).
Namzaric
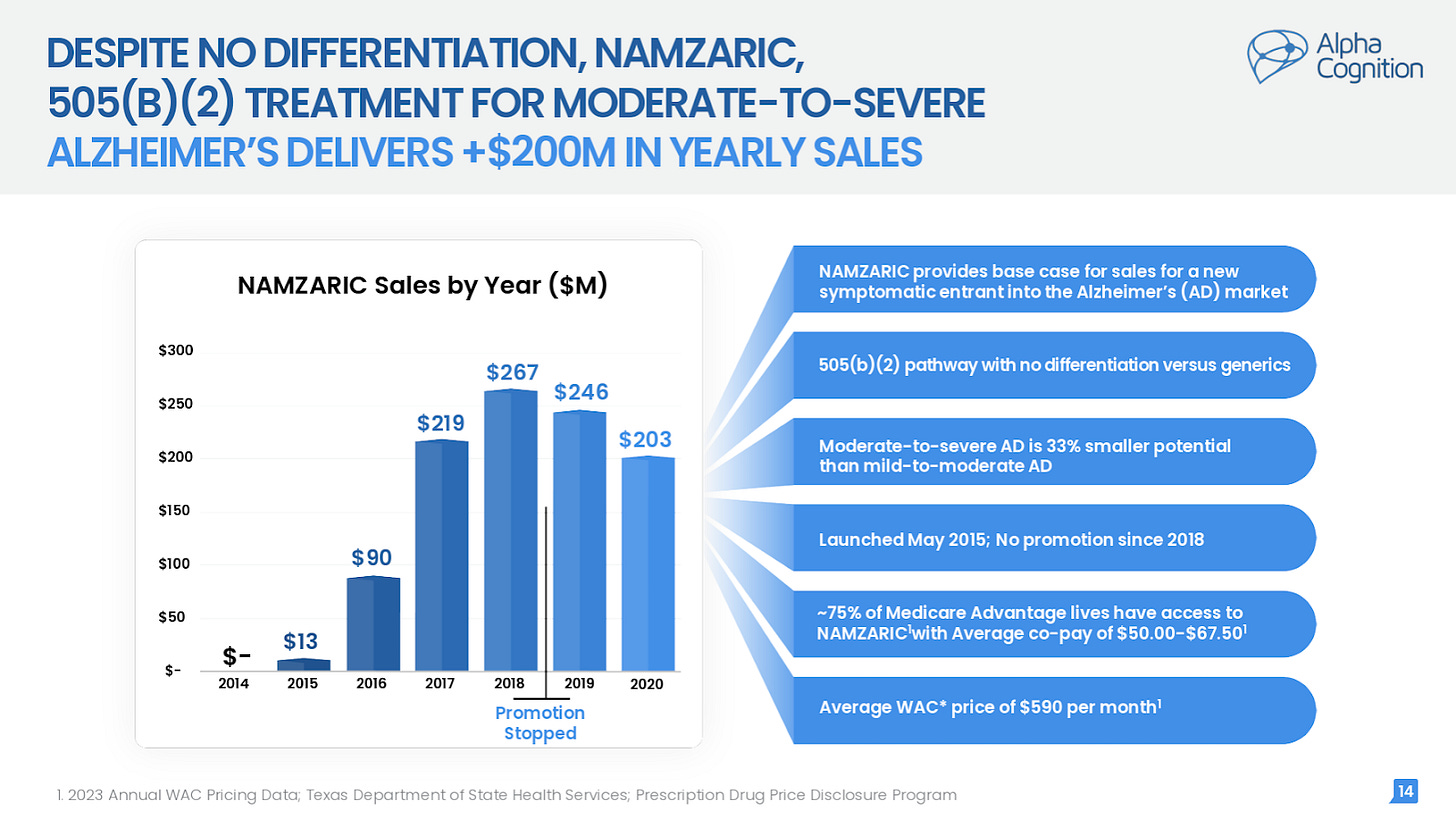
Namzaric, like Zunveyl, was approved under the FDA’s 505(b)(2) pathway as a simple co-formulation of existing drugs donepezil and memantine. Despite being a simple fixed-dose combination that doctors could easily prescribe separately, i.e. no differentiation, Namzaric went on to reach $267 million of peak sales in 2018.
Alpha Cognition states that Namzaric continues to deliver $200+ million of sales despite ceasing promotion. Namzaric also still has ~70% insurance coverage and is priced at ~$670/month (again, despite zero differentiation versus generic donepezil + memantine).
Reminyl/Razadyne (galantamine)
Even the original galantamine, which had the worst side effect profile of all AChEIs and was last to market, was able to reach ~$250 million of peak sales. If Zunveyl essentially solves AChEI tolerability issues and unlocks galantamine’s underlying efficacy as we believe it should, it should be able to do multiples of galantamine’s $250 million peak sales number.
Revenue Model
While it is hard to make concrete forecasts about the company’s near term revenue ramp between physician education/adoption, payor hurdles, and lack of guidance from management, we feel relatively confident in projecting a significant revenue acceleration in 2026 and 2027, as the CEO has alluded to.
2025: We think it is reasonable to assume something like $5-10 million of cumulative revenue for 2025, primarily 4Q25-loaded as the company penetrates more LTC facilities/networks and doctors try Zunveyl on a small number of their patients.
2026: We think revenue could rise to something like $25-30 million of sales in 2026, again ramping throughout the year as physicians, assuming positive initial experiences with Zunveyl, prescribe more patients, and as the payor landscape potentially gets easier for prescribers. For context, this would represent only ~3k whole-year-patient equivalents on Zunveyl, which is well less than 1% of the estimated LTC Alzheimer’s patient population.
2027: Though management guides to breakeven in this year, we think the company could meaningfully exceed the implied ~$40 million of revenue and do something like $60 million of sales in 2027, which would represent ~100% growth over 2025 and would still only represent an average of ~7k patients on Zunveyl in 2027 (assuming full WAC of $749/month).
Valuation
At $40-60 million of 2027 sales, and with an expectation that revenue growth should remain strong in 2028 and beyond, we think Alpha Cognition could fetch anything from a 5x to 10x sales multiple, which would imply a market cap of $250-$500 million at the midpoint of our 2027 revenue forecast, which represents roughly 100%-400% upside versus the current market cap of $120 million.
We also believe there could be upside to our valuation analysis both on a revenue/profitability/execution basis, but also on a multiple basis as Alpha Cognition represents a rather unique opportunity as a differentiated and unopposed new entrant to the large, established, and underserved Alzheimer’s market.
Financial Position
With $49 million of cash as of 12/31/24 and $6 million received/anticipated from the out-licensing to CMS Pharma in Southeast Asian territories, the company has stated that it believes it can reach breakeven status with its current cash position. This guidance factors in expense guidance of $38-42 million in 2025 (and presumably similar in 2026 and 2027).
With ~$40 million of expenses expected in 2025, the company will need to ramp revenue reasonably quickly in order to reach breakeven before it needs to raise additional capital.
Capitalization and Ownership
The company has 16 million basic and 22 million fully-diluted shares outstanding.
Alpha Cognition’s $50 million equity offering in November 2024 included a solid proportion of institutional buyers. As of the latest filings, 47% of shares are owned by hedge funds or other institutional investors, a pretty high proportion for micro cap biotech. We will see what happens when ownership filings are updated. The largest holders are Solas Capital Management, AWM Investment Company, and Cable Car Capital, all of which participated in the equity offering.
Risks to the Thesis
Disease Modifying Alzheimer’s Development
Perhaps the biggest risk to Alpha Cognition’s long-term story is that another company comes up with disease-modifying therapy for Alzheimer’s. If, for instance, a company like Inmune Bio, which has a Phase 2 readout in June for its inflammation-targeting Alzheimer’s drug, reports either disease stabilization or actual cognitive improvement, Alpha Cognition’s medium- and long-term market potential would have to be tempered to some degree.
Zunveyl 505(b)(2) Pathway
Since Zunveyl was approved via the 505(b)(2) pathway, its bioequivalence studies were conducted in healthy volunteers, which means the ~2% rate of GI side effects and zero incidences of insomnia could be different when translating to Alzheimer’s patients. Also, some physicians may be less readily willing to prescribe Zunveyl because of its 505(b)(2) status and lack of direct testing in Alzheimer’s patients, even though the data on galantamine is very robust. Some physicians may also be relatively unaware of galantamine’s efficacy data because of how popular Aricept is.
Physician Education
It may take longer than expected to educate physicians in LTC facilities across the country, especially with a relatively small team. While the team has excellent experience in the space, we are not acutely familiar enough with the LTC space to know what adoption hurdles or inefficiencies may stand in the way.
Avanir Pharmaceuticals Debacle
A number of team members at Alpha Cognition, including the CEO and CCO/COO, previously worked at Avanir Pharmaceuticals, which was accused of promoting off-label use of Nuedexta and paying doctors kickbacks. Avanir eventually paid over $100 million in a settlement in 2019. While Avanir may have used aggressive sales tactics, this doesn’t turn us away from the story.
Dilution Risk
While the company believes it will be able to reach breakeven with its current cash on hand, a number of circumstances could lead to a capital raise before 2027, whether to develop pipeline opportunities or because product adoption is lagging.
Conclusion
Alpha Cognition is an underknown, clinically de-risked biopharma launching a novel and differentiated drug into a large and woefully underserved Alzheimer’s market, with essentially no competition from other branded products. And while the company is essentially completely unknown at the moment, that could change quickly once its starts to report real revenue in later 2025 and especially in 2026. With management’s guidance to breakeven in ~2027 implying a $40 million annual run rate which we believe could prove to be quite conservative, Alpha Cognition could easily trade at multiple times its current $120 million valuation over the next couple years. While disease-modifying therapies are a valid concern over the medium and longer term, we are optimistic about Zunveyl’s potential to become the new best-in-class symptomatic treatment for Alzheimer’s patients, taking the blockbuster throne from donepezil and delivering improved outcomes for patients, healthcare providers, and family members.